CD20-Targeted Antibody, Rituxan, Sanctioned for Prevention and Therapy of Organ Rejection Due to Antibodies
Zenyaku Kogyo Co., Ltd., in collaboration with Chugai Pharmaceutical Co., Ltd., has publicly disclosed that it has successfully received the necessary authorization from Japan's Ministry of Health, Labour and Welfare. This endorsement pertains to the commercial distribution of a biologic drug known as Rituxan®, which is administered via intravenous infusion, available in two dosage strengths: 100 mg and 500 mg. The authorized generic drug, rituximab, is specifically indicated for the control and therapeutic management of antibody-mediated rejection incidents following organ transplant procedures.
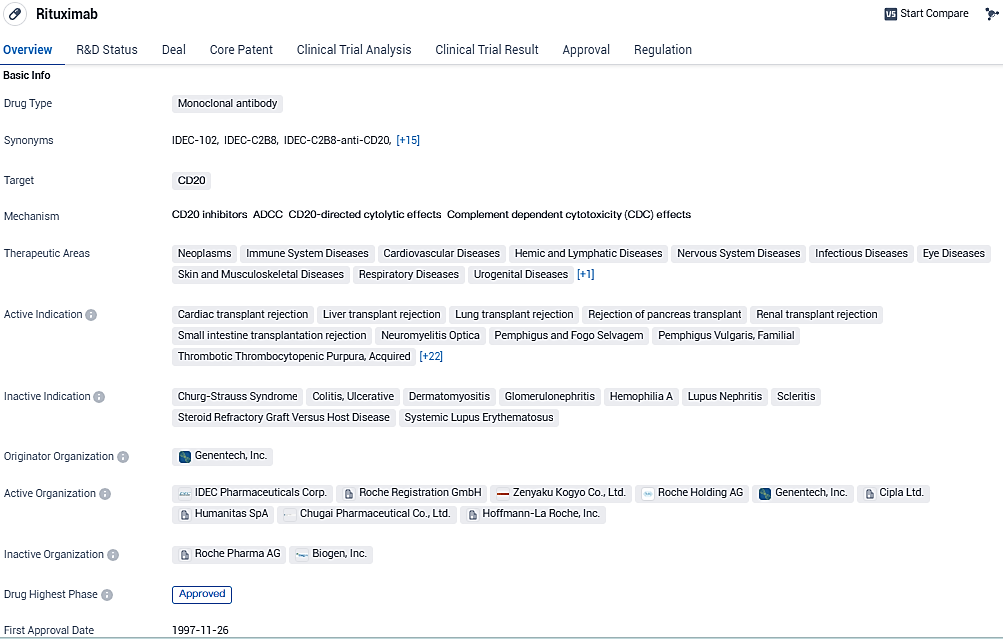
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic drug Rituxan is recognized for its ability to lock onto CD20, an antigen located on the surface of B lymphocytes, while not affecting the progenitor cells of blood nor the mature plasma cells. It utilizes the existing immune machinery within humans to target and incapacitate these specific B cells. By hindering the maturation process of these cells into entities that produce antibodies, it has a potential role in reducing antibody generation against transplanted tissues.
Rituxan's journey toward being a candidate for the "management and intervention of antibody-mediated organ transplant rejection" was initiated following insights from the "34th Conclave on Drugs Without Approval and Off-label Use for Critical Health Demands." The impetus for its study emerged during the third invitation for nominations of drugs requiring approval or those that were used off-label, which began in August 2013. During this phase, the Japan Society of Transplantation proposed the development of therapies focused on the "management of antibody-mediated rejection post-kidney transplant" and for "pre-transplant decensitizing protocols in patients with antibodies against the kidney donor."
Utilizing the findings from these investigations, Zenyaku put forth a petition on April 6, 2023, for amendments to their existing licenses concerning the production and commercialization of the drug, ultimately obtaining the recent endorsement.
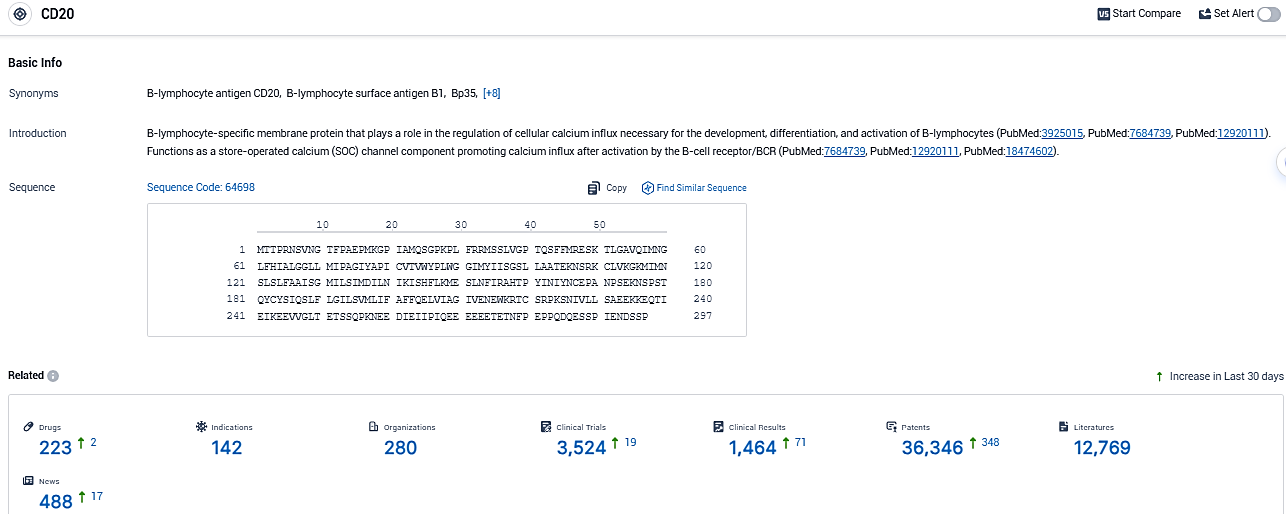
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of December 30, 2023, there are 223 investigational drugs for the CD20 target, including 142 indications, 280 R&D institutions involved, with related clinical trials reaching 3524, and as many as 12769 patents.
Rituximab is a monoclonal antibody drug that targets CD20 and has been approved for the treatment of various diseases across multiple therapeutic areas. It was first approved in the United States in 1997 and is classified as an orphan drug. Its wide range of indications highlights its versatility and potential impact in the field of biomedicine.