UCB announces EU approval for RYSTIGGO as an adult generalized myasthenia gravis treatment
UCB, an international enterprise in the biopharmaceutical sector, revealed that on January 5th, 2024, the European Commission sanctioned the commercial distribution of RYSTIGGO® (rozanolixizumab), approving its use in combination with conventional treatments. This endorsement specifically pertains to administering care to adult patients diagnosed with generalized myasthenia gravis who have tested positive for either anti-acetylcholine receptor antibodies or anti-muscle-specific tyrosine kinase antibodies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
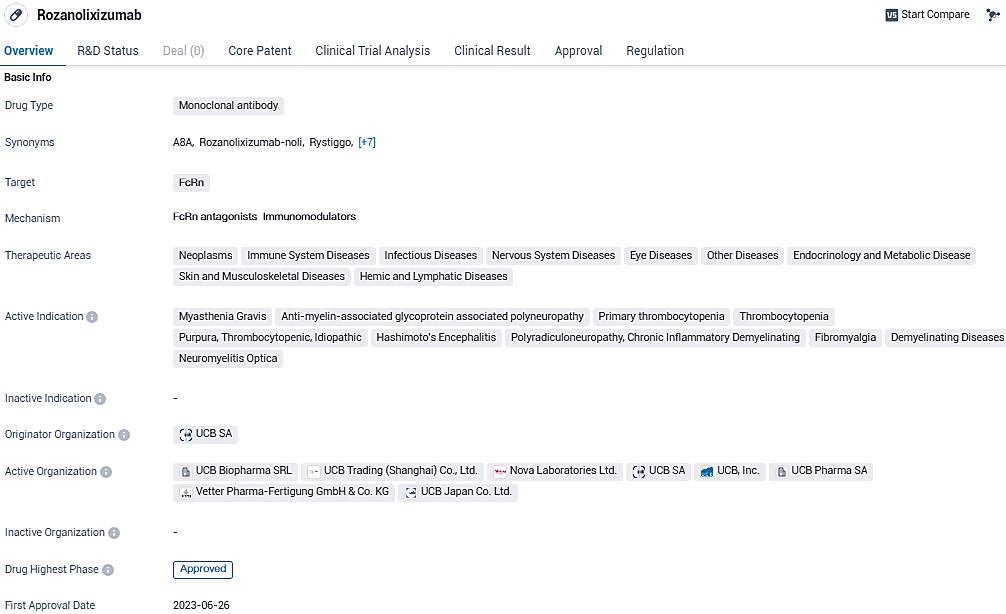
The injectable solution of rozanolixizumab at a concentration of 140 mg/ml is a humanized antibody of the IgG4 subclass that targets the neonatal Fc receptor, FcRn. This interaction leads to a decrease in the levels of IgG antibodies in the bloodstream. Notably, it has been authorized in Europe as the initial treatment option for adult patients suffering from myasthenia gravis (gMG) who test positive for either anti-AChR or anti-MuSK antibodies—the two predominant variants of gMG.
As of December 2023, the European Commission sanctioned the use of UCB's ZILBRYSQ® (zilucoplan) as a supplemental therapy in combination with established treatments for adult individuals with gMG who are confirmed to be anti-AChR antibody-positive. Administered once daily through subcutaneous self-injection, zilucoplan is a peptide-based inhibitor that specifically acts on the complement component 5.
UCB is actively developing a diverse range of medical treatments for managing gMG, with the strategic goal of equipping healthcare professionals with alternatives to target either the complement system or pathological antibodies, tailored to the needs of their patients. UCB's commitment to fulfilling the unmet demands within the gMG community is manifested through the creation of such a varied and dedicated product lineup.
Jean-Christophe Tellier, the CEO of UCB, announced with enthusiasm the clearance of rozanolixizumab by the European Commission, a certification that comes on the heels of the approval of zilucoplan. He noted that this progress is a vital stride in UCB's pledge to enhance therapeutic options for patients with gMG in Europe, contributing to the sustained advancement of UCB’s product introductions in the medical field.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
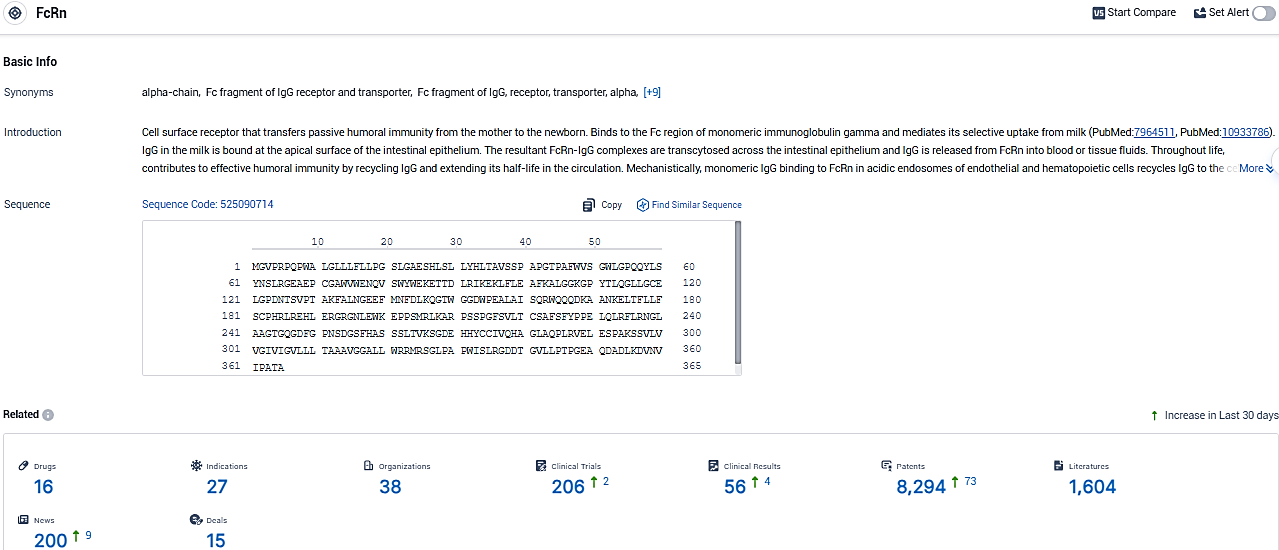
According to the data provided by the Synapse Database, As of January 12, 2024, there are 16 investigational drugs for the FcRn target, including 27 indications, 38 R&D institutions involved, with related clinical trials reaching 206, and as many as 8294 patents.
The approval of rozanolixizumab from the EC is valid in all EU member states, as well as in the European Economic Area countries Iceland, Liechtenstein, and Norway. Orphan designation was granted by the European Commission in 2020 to rozanolixizumab for the treatment of myasthenia gravis and maintained after having received the positive CHMP Opinion.