UCB Reveals Positive Findings on Bepranemab for Early Alzheimer’s in Phase 2a Trial at CTAD 2024
UCB presented Phase 2a findings from the TOGETHER (AH0003) trial, which explored the safety, effectiveness, and tolerability of bepranemab, an experimental anti-tau antibody aimed at the central portion of the tau protein, in individuals experiencing prodromal to mild Alzheimer's disease. These results were shared during a late-breaking session at the 2024 Clinical Trials on Alzheimer’s Disease (CTAD) conference.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"We find great encouragement in the proof-of-concept results for bepranemab, which emphasize its potential to influence the progression of early Alzheimer’s disease. This reinforces our conviction that targeting the mid-region of tau represents a critical approach for changing the course of the disease," stated Alistair Henry, Chief Scientific Officer at UCB. "We are sincerely grateful to the patients, their families, and the committed clinical trial teams who have collaborated with us in this vital research. Their support is crucial as we investigate the subsequent phases of bepranemab’s development."
Total Study Population
In the total study cohort, there were no significant benefits noted from either low or high doses of bepranemab compared to placebo on the primary endpoint, which is the Clinical Dementia Rating Scale Sum of Boxes (CDR-SB) total score at Week 80 (CDR-SB serves as an assessment of cognitive function). Nevertheless, at Week 80, in several key secondary outcomes, treatment with bepranemab (45mg/kg and 90mg/kg):
- Reduced the rate of tau accumulation in major brain regions by 33%-58% compared to placebo.
- Diminished cognitive decline by 21%-25% relative to placebo, based on the change in the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog14) total score from Baseline to Week 80.
Consistent Treatment Effects in Subgroup Analysis
In two specified subgroups, one with low tau burden at Baseline and the other with APOε4* non-carriers, benefits from treatment were noted across various outcome measures. A post-hoc analysis of participants with low tau at Baseline or those who are APOε4 non-carriers (representing about 50% of the overall cohort) indicated that high-dose bepranemab (90mg/kg):
- Decreased the rate of tau accumulation in significant brain areas by 63%-67% compared to placebo at Week 80.
- Slowed clinical disease progression by 29% based on the changes in CDR-SB from Baseline to Week 80.
- Reduced disease progression in secondary/exploratory measures, including Activities of Daily Living assessments by 41%-54% by Week 80.
Conversely, in the subgroup of participants with high tau at Baseline who were APOε4 carriers, high-dose bepranemab demonstrated minimal benefits across nearly all clinical outcomes, with CDR-SB, A-iADL-Q, and ADCS-ADL scores showing a preference for the placebo group.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
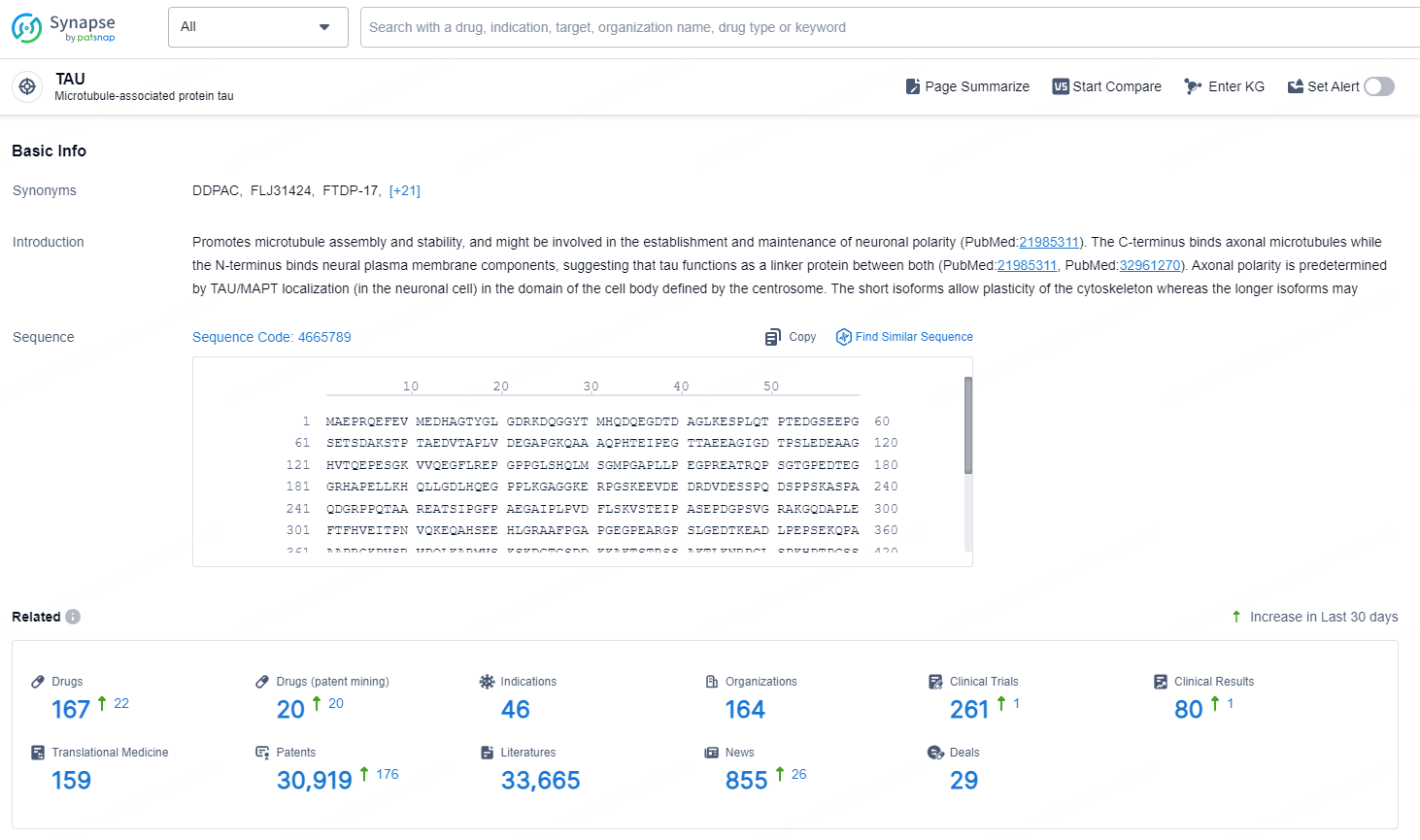
According to the data provided by the Synapse Chemical, As of November 5, 2024, there are 167 investigational drugs for the TAU target, including 46 indications, 164 R&D institutions involved, with related clinical trials reaching 261, and as many as 30919 patents.
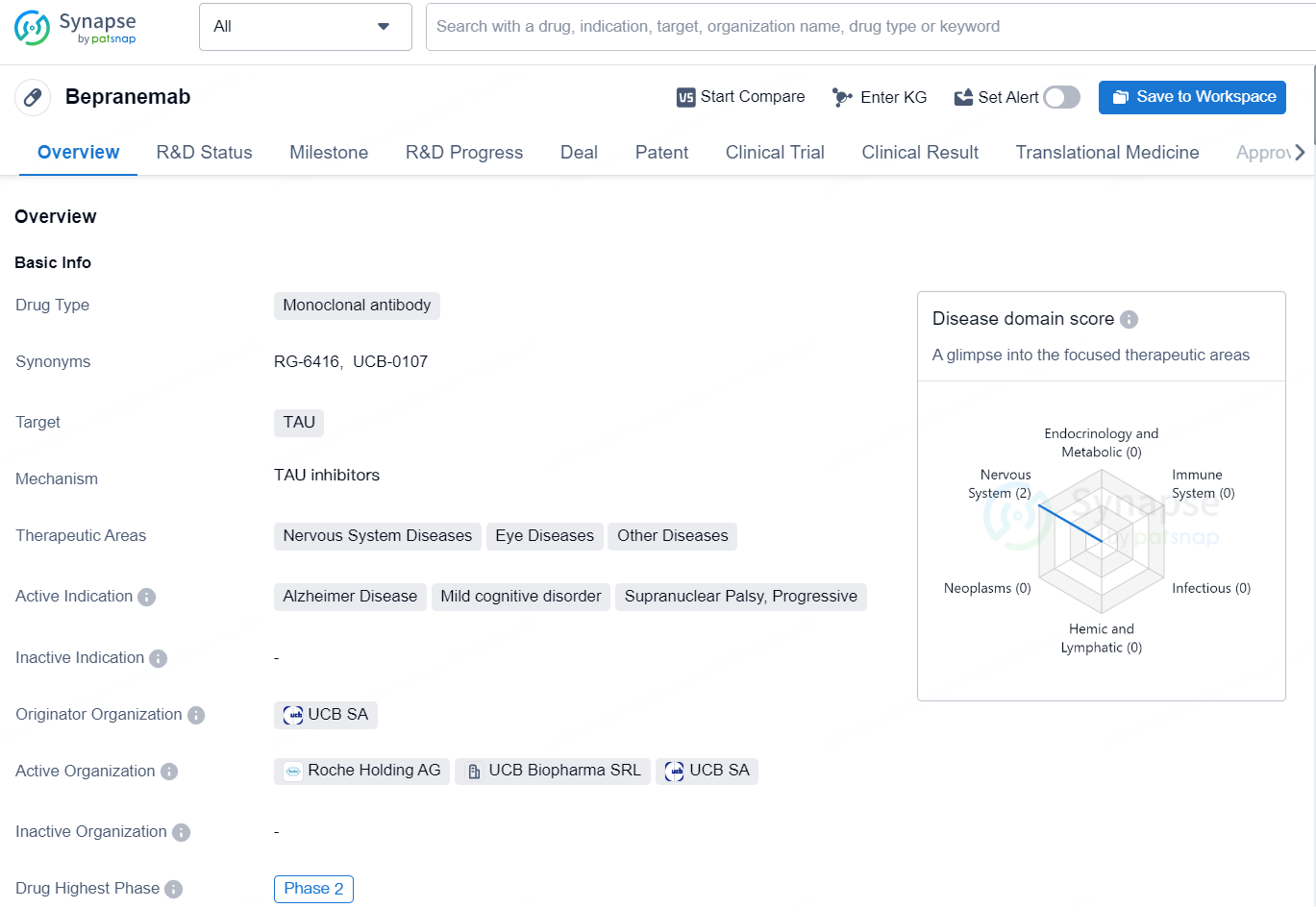
Bepranemab is a monoclonal antibody drug that targets TAU, a protein associated with various nervous system diseases, eye diseases, and other conditions. The drug is intended for the treatment of Alzheimer's Disease, mild cognitive disorder, and other neurodegenerative conditions such as supranuclear palsy and progressive.