Unleashing the Power of Brompheniramine maleate: A Comprehensive Review on R&D Breakthroughs
Brompheniramine maleate's R&D Progress
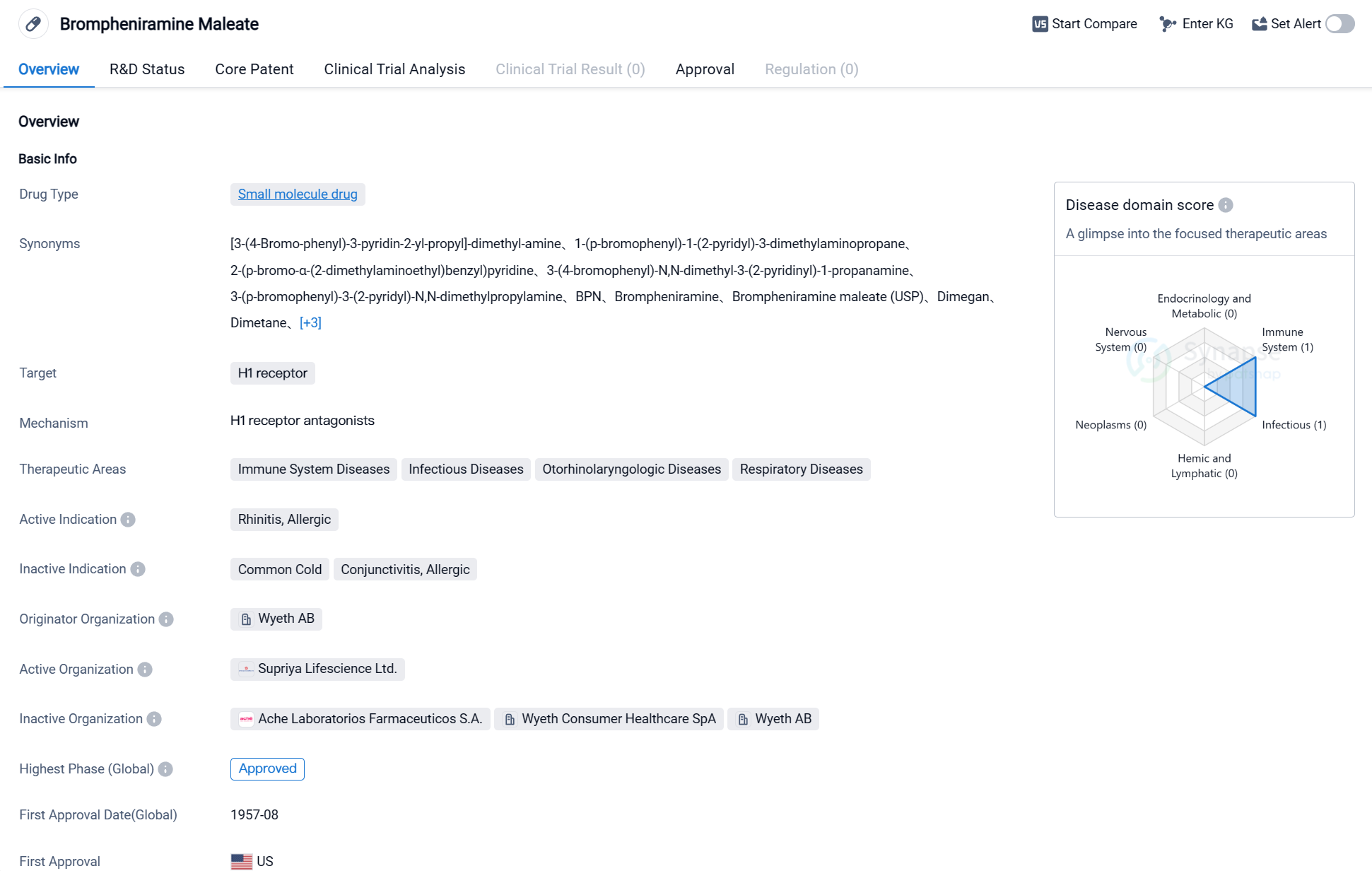
Brompheniramine Maleate is a small molecule drug that acts as an antagonist of the H1 receptor. It is primarily used in the treatment of various conditions related to the immune system, infectious diseases, otorhinolaryngologic diseases, and respiratory diseases. The active indication for this drug is allergic rhinitis.
The drug was first approved in the United States in August 1957, making it a well-established medication with a long history of use. It is important to note that the information provided does not specify the current approval status in other countries, but it is mentioned that the highest phase of development for this drug is approved globally.
The originator organization of Brompheniramine Maleate is Wyeth AB, a pharmaceutical company known for its contributions to the development of various medications. The highest R&D phase of this drug is approved.
Brompheniramine Maleate is primarily indicated for the treatment of allergic rhinitis, a condition characterized by inflammation of the nasal passages due to an allergic reaction. By targeting the H1 receptor, this drug helps alleviate symptoms such as sneezing, itching, and a runny nose associated with allergic rhinitis.
The drug's small molecule nature suggests that it is a chemically synthesized compound rather than a biologic or natural product. Small molecule drugs are typically easier to manufacture and have a well-defined chemical structure, allowing for better control over their pharmacokinetics and pharmacodynamics.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Brompheniramine maleate: H1 receptor antagonists
H1 receptor antagonists, also known as H1 blockers, are a class of drugs that block the action of histamine at the H1 receptors. Histamine is a chemical released by the body during an allergic reaction, causing symptoms such as itching, sneezing, runny nose, and watery eyes. H1 receptor antagonists work by binding to the H1 receptors and preventing histamine from binding to these receptors, thereby reducing or preventing the allergic response.
From a biomedical perspective, H1 receptor antagonists are commonly used in the treatment of allergic conditions, such as allergic rhinitis (hay fever), urticaria (hives), and allergic conjunctivitis. They can help alleviate symptoms such as itching, sneezing, and nasal congestion by blocking the effects of histamine. Some examples of H1 receptor antagonists include cetirizine, loratadine, and fexofenadine, which are available over-the-counter or by prescription. These drugs are generally well-tolerated and have a good safety profile, although they may cause drowsiness in some individuals. It is important to follow the recommended dosage and consult a healthcare professional if symptoms persist or worsen.
Drug Target R&D Trends for Brompheniramine maleate
According to Patsnap Synapse, as of 2 Sep 2023, there are a total of 268 H1 receptor drugs worldwide, from 280 organizations, covering 131 indications, and conducting 1694 clinical trials.
The analysis of the target H1 receptor reveals a competitive landscape with several companies showing significant growth and progress. GSK Plc, Sanofi, and Bayer AG are among the companies with the highest stage of development. The indications with the highest number of approved drugs include Rhinitis, Allergic, and Common Cold. Small molecule drugs are the most rapidly progressing drug type under the target H1 receptor. China is the country developing the fastest in this area, with a large number of approved drugs. Overall, the target H1 receptor presents opportunities for further research and development, particularly in the areas of allergic and inflammatory conditions.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Brompheniramine Maleate is a small molecule drug that targets the H1 receptor and is primarily used in the treatment of allergic rhinitis. It has been approved for use in the United States since 1957 and is indicated for various conditions related to the immune system, infectious diseases, otorhinolaryngologic diseases, and respiratory diseases. The drug was developed by Wyeth AB and has reached the highest phase of approval globally.