Unleashing the Power of Dicloxacillin sodium: A Comprehensive Review on R&D Breakthroughs
Dicloxacillin sodium's R&D Progress
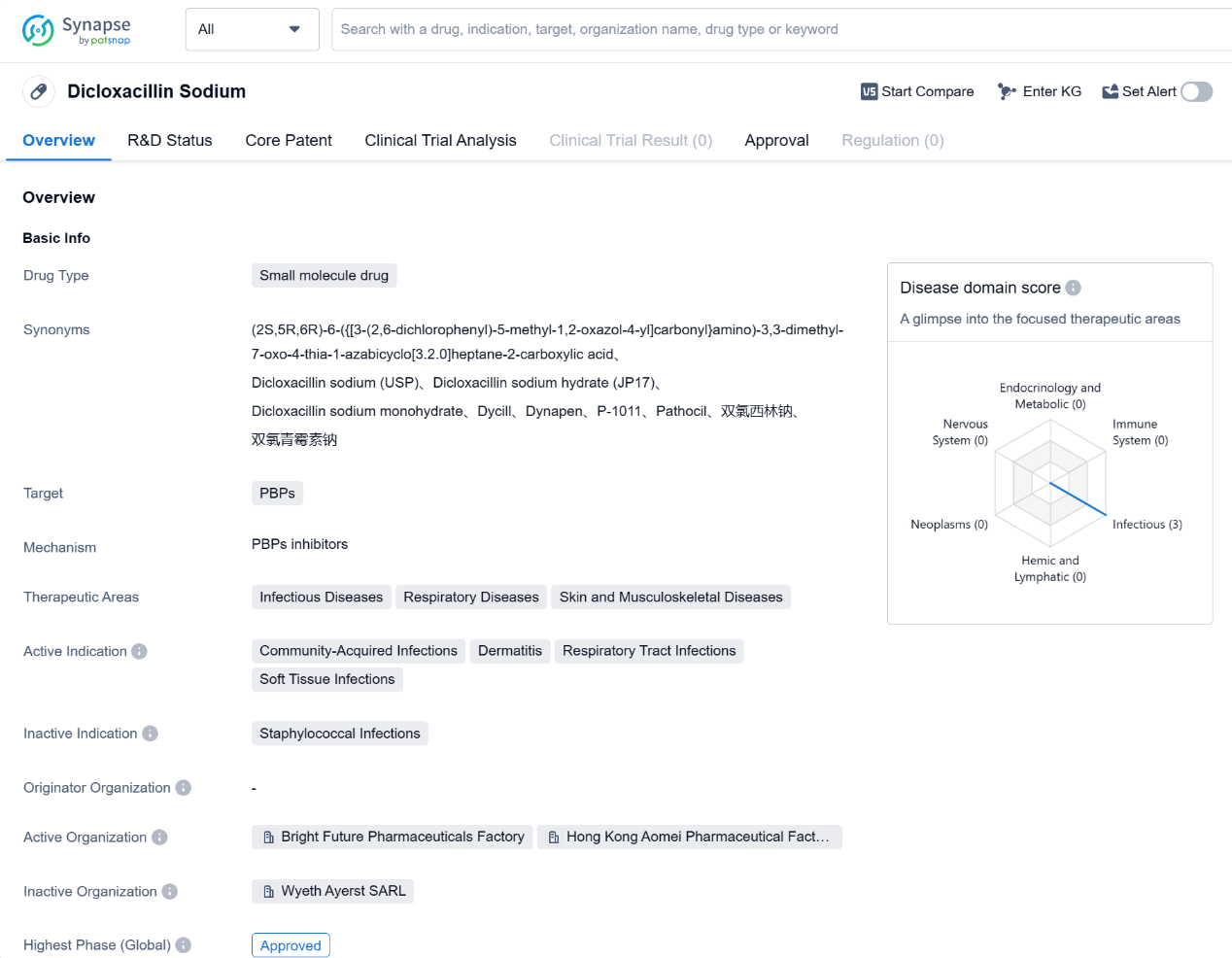
Dicloxacillin Sodium is a small molecule drug that belongs to the class of penicillin antibiotics. It primarily targetsPenicillin-Binding Proteins (PBPs) to exert its therapeutic effects. The drug is mainly used in the treatment of various infectious diseases, respiratory diseases, and skin and musculoskeletal diseases.
Dicloxacillin Sodium has been approved for use in the treatment of community-acquired infections, dermatitis, respiratory tract infections, and soft tissue infections. These indications cover a wide range of conditions caused by bacterial infections, including those affecting the skin, respiratory system, and musculoskeletal system. The drug is particularly effective against infections caused by gram-positive bacteria, including Staphylococcus aureus.
The highest phase of development for Dicloxacillin Sodium is approved, indicating that it has successfully completed clinical trials and has been granted regulatory approval for use. This approval status applies both globally and in China. The drug received its first approval in the United States in April 1968, making it a well-established and long-standing treatment option.
Dicloxacillin Sodium has been widely used for several decades and has proven to be a reliable and effective antibiotic in the treatment of various bacterial infections. Its mechanism of action, targeting PBPs, allows it to inhibit the synthesis of bacterial cell walls, leading to the destruction of the bacteria.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Dicloxacillin sodium: PBPs inhibitors
PBPs inhibitors are a type of drugs that target penicillin-binding proteins (PBPs). These proteins are enzymes involved in the synthesis of bacterial cell walls. PBPs inhibitors work by binding to the active site of PBPs, preventing them from carrying out their normal function of cross-linking the peptidoglycan strands in the bacterial cell wall. This leads to weakened cell walls and ultimately the death of the bacteria. PBPs inhibitors are commonly used in the treatment of bacterial infections and are particularly effective against gram-positive bacteria. They are a crucial class of antibiotics and play a significant role in combating bacterial resistance.
Drug Target R&D Trends for Dicloxacillin sodium
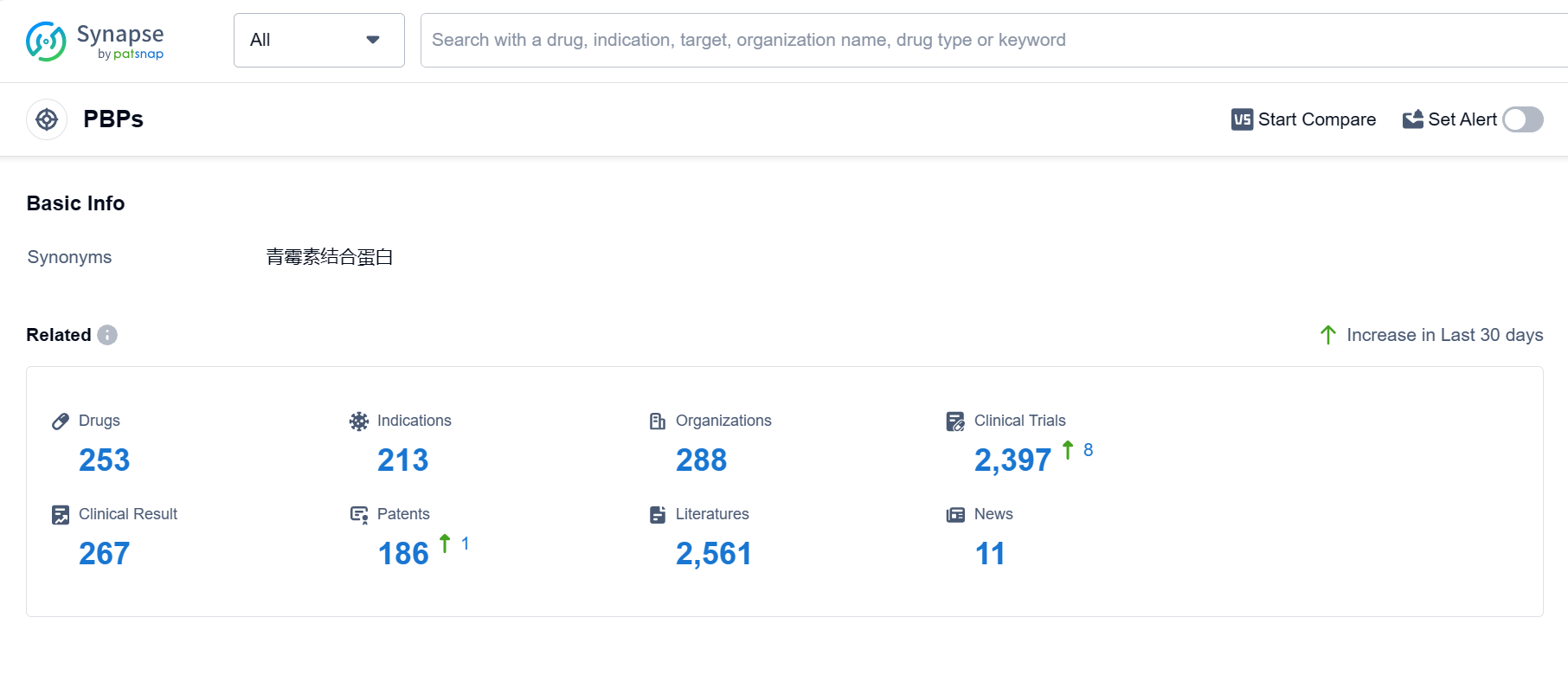
PBPs, or Penicillin-Binding Proteins, play a crucial role in the human body. These proteins are found in the cell walls of bacteria and are responsible for the synthesis and maintenance of the peptidoglycan layer, a vital component of bacterial cell walls. PBPs act as targets for antibiotics, particularly beta-lactam antibiotics like penicillin, by binding to them and inhibiting the enzymes that cross-link the peptidoglycan strands. This disruption weakens the bacterial cell wall, leading to cell lysis and ultimately bacterial death. Understanding the role of PBPs is essential in developing effective antibiotics and combating bacterial infections.
According to PatSnap Synapse, as of 4 Sep 2023, there are a total of 253 PBPs drugs worldwide, from 288 organizations, covering 213 indications, and conducting 2397 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, Dicloxacillin Sodium is a small molecule drug that targets PBPs and is approved for the treatment of infectious diseases, respiratory diseases, and skin and musculoskeletal diseases. It has a long history of use, with its first approval dating back to 1968 in the United States. The drug's effectiveness against gram-positive bacteria and its established safety profile make it a valuable option in the treatment of bacterial infections.