Unleashing the Power of Idoxuridine: A Comprehensive Review on R&D Breakthroughs
Idoxuridine's R&D Progress
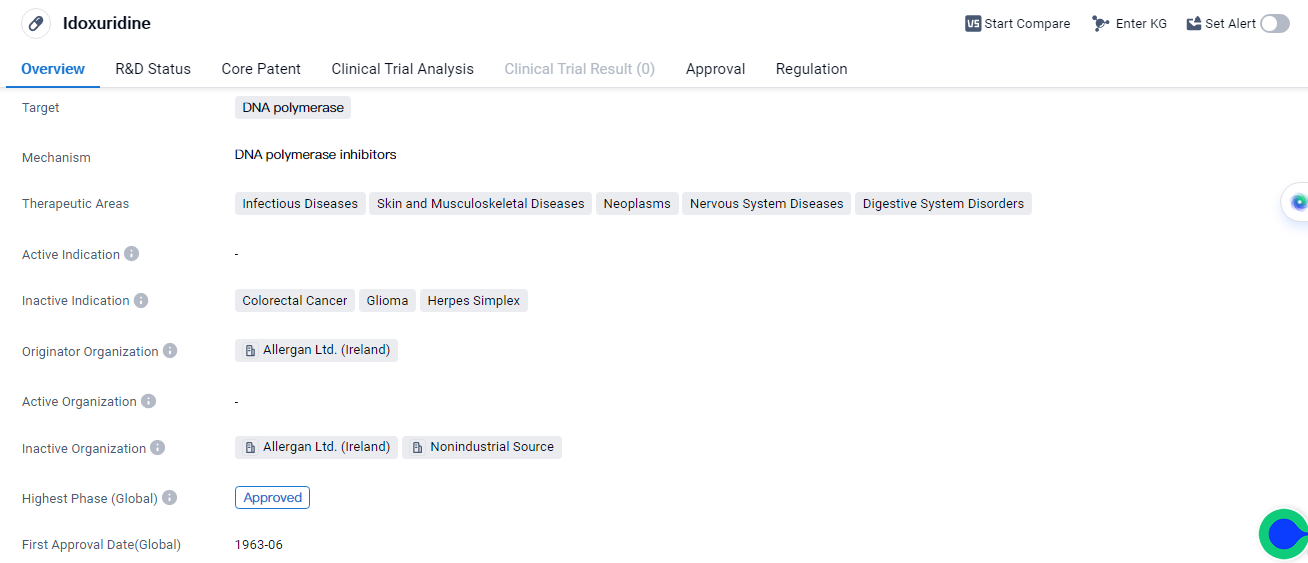
Idoxuridine is a small molecule drug that targets DNA polymerase. It has been approved for treatment of infectious diseases, skin and musculoskeletal diseases, neoplasms, nervous system diseases, and digestive system disorders, etc. The drug was first approved in the United States in June 1963 and is regulated as an orphan drug.
Idoxuridine is developed by Allergan Ltd., an organization based in Ireland. It has reached the highest phase of approval globally. As a small molecule drug, Idoxuridine works by inhibiting the activity of DNA polymerase, an enzyme involved in DNA replication. By targeting this enzyme, Idoxuridine can interfere with the replication of viral DNA, making it effective against infectious diseases caused by DNA viruses.
The therapeutic areas in which Idoxuridine is approved indicate its broad spectrum of activity. It can be used to treat various infectious diseases which affect the skin and musculoskeletal system. Additionally, Idoxuridine has shown efficacy in the treatment of neoplasms, nervous system diseases, and digestive system disorders. This suggests its potential in addressing a wide range of medical conditions.
The fact that Idoxuridine is regulated as an orphan drug highlights its importance in treating rare diseases or conditions that affect a small number of patients. Orphan drugs often receive special regulatory incentives and exclusivity periods to encourage their development and availability for patients with unmet medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Idoxuridine: DNA polymerase inhibitors
DNA polymerase inhibitors are a class of drugs that inhibit the activity of DNA polymerase enzymes. DNA polymerases are essential enzymes involved in DNA replication, repair, and synthesis. These inhibitors work by blocking the action of DNA polymerase, preventing it from adding nucleotides to the growing DNA chain.
In a biomedical perspective, DNA polymerase inhibitors are used in the field of biomedicine to treat various diseases, particularly viral infections. Viruses often rely on DNA polymerase enzymes to replicate their genetic material, and by inhibiting these enzymes, the replication process can be disrupted, preventing the virus from multiplying and spreading.
These inhibitors are commonly used in antiviral therapies, targeting specific viral DNA polymerases. By inhibiting viral DNA polymerase, the replication of the viral genome is halted, reducing the viral load and allowing the immune system to effectively eliminate the infection.
It is important to note that DNA polymerase inhibitors can also affect human DNA polymerases, which can lead to side effects. Therefore, the development of DNA polymerase inhibitors requires careful consideration of their selectivity and potential toxicity to human cells.
Drug Target R&D Trends for Idoxuridine
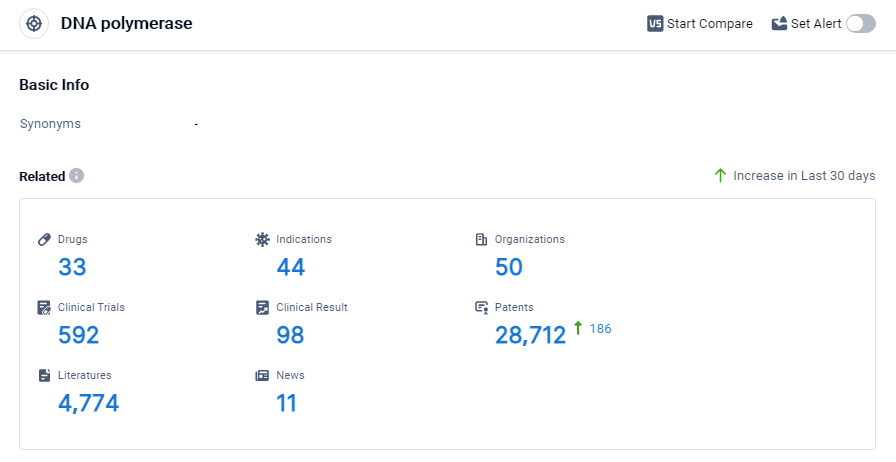
According to Patsnap Synapse, as of 3 Sep 2023, there are a total of 33 DNA polymerase drugs worldwide, from 50 organizations, covering 44 indications, and conducting 592 clinical trials.
The analysis of the current competitive landscape and future development of target DNA polymerase reveals that GSK plc, Gilead Sciences, Inc., and Sino Biopharmaceutical Ltd. are the companies with the highest stage of development. These companies have made significant progress in R&D, particularly in the development of small molecule drugs. The approved indications for drugs targeting DNA polymerase include hepatitis B, herpes simplex, leukemia, and various viral infections. China, Japan, and the United States are the countries/locations with the highest development progress, with China leading the way. The future development of target DNA polymerase is expected to focus on the development of small molecule drugs, with intense competition in the market. China's progress in this field highlights its growing influence in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Idoxuridine is a small molecule drug developed by Allergan Ltd. It targets DNA polymerase and has been approved for the treatment of infectious diseases, skin and musculoskeletal diseases, neoplasms, nervous system diseases, and digestive system disorders. It was first approved in the United States in 1963 and is regulated as an orphan drug. Its broad spectrum of activity and potential in treating rare diseases make it a valuable asset in the field of biomedicine.