Unleashing the Power of nicardipine hydrochloride: A Comprehensive Review on R&D Breakthroughs
Nicardipine hydrochloride's R&D Progress
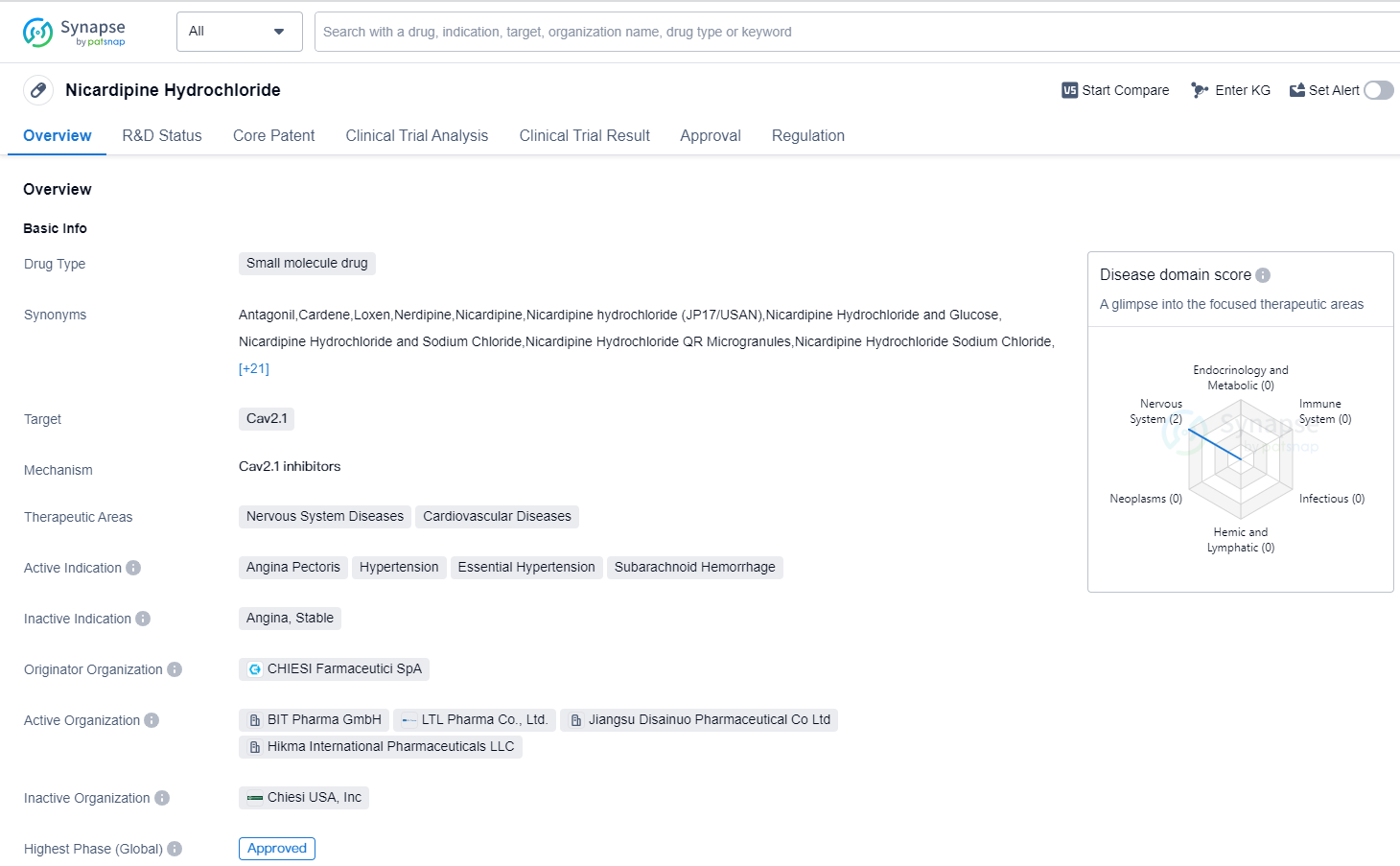
Nicardipine Hydrochloride is a small molecule drug that targets Cav2.1 and is used in the treatment of various nervous system and cardiovascular diseases. It has been approved for use in the treatment of angina pectoris, hypertension, essential hypertension, and subarachnoid hemorrhage. The drug was first approved in Japan in May 1981 and is regulated as an orphan drug.
Nicardipine Hydrochloride is developed by CHIESI Farmaceutici SpA, an originator organization in the pharmaceutical industry. As a small molecule drug, it is designed to interact with Cav2.1, a target involved in the regulation of calcium channels. By targeting this specific protein, Nicardipine Hydrochloride aims to modulate calcium influx, which plays a crucial role in various physiological processes.
The therapeutic areas of Nicardipine Hydrochloride include nervous system diseases and cardiovascular diseases. These conditions encompass a wide range of disorders affecting the brain, spinal cord, and peripheral nerves, as well as conditions related to the heart and blood vessels. By targeting Cav2.1, Nicardipine Hydrochloride may help alleviate symptoms and improve outcomes in patients with these diseases.
Nicardipine Hydrochloride has received approval for use in multiple indications. Angina pectoris, a condition characterized by chest pain or discomfort due to reduced blood flow to the heart, is one of the approved indications. Hypertension, or high blood pressure, is another condition for which Nicardipine Hydrochloride is approved. Additionally, it is indicated for the treatment of essential hypertension, which refers to high blood pressure with no identifiable cause, and subarachnoid hemorrhage, a type of stroke caused by bleeding into the space surrounding the brain.
The drug's first approval in Japan in 1981 indicates its long history of use in the global market. It has also received approval in China, further expanding its reach.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for nicardipine hydrochloride: Cav2.1 inhibitors
Cav2.1 inhibitors are a type of drugs that target and inhibit the activity of the Cav2.1 calcium channels. These channels, also known as P/Q-type calcium channels, are found in the central nervous system and play a crucial role in regulating neurotransmitter release. By inhibiting the Cav2.1 channels, Cav2.1 inhibitors can modulate the release of neurotransmitters such as glutamate and GABA, which are involved in various neurological processes.
From a biomedical perspective, Cav2.1 inhibitors have potential therapeutic applications in the treatment of neurological disorders such as epilepsy, migraine, and chronic pain. By reducing the excessive release of neurotransmitters, these inhibitors can help regulate neuronal excitability and alleviate symptoms associated with these conditions.
Drug Target R&D Trends for nicardipine hydrochloride
Cav2.1, also known as P/Q-type calcium channels, plays a crucial role in the human body. These channels are primarily found in the central nervous system and are responsible for regulating the release of neurotransmitters. By controlling the influx of calcium ions into neurons, Cav2.1 channels contribute to the transmission of signals between nerve cells, ultimately influencing various physiological processes such as muscle contraction, hormone secretion, and synaptic plasticity. Dysregulation or dysfunction of Cav2.1 channels has been associated with neurological disorders like epilepsy, ataxia, and migraine. Understanding the role of Cav2.1 channels provides valuable insights for the development of therapeutic interventions targeting these channels to treat related disorders.
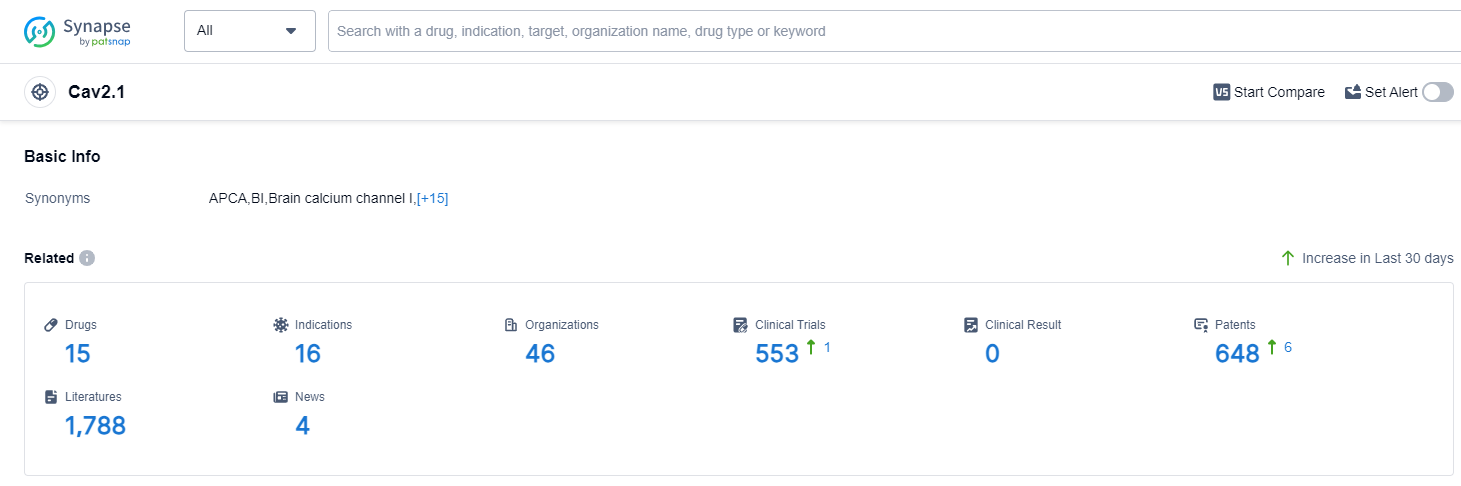
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 15 Cav2.1 drugs worldwide, from 46 organizations, covering 16 indications, and conducting 553 clinical trials.
The analysis of the current competitive landscape and future development of target Cav2.1 reveals several key findings. Bayer AG, LTL Pharma Co., Ltd., and CHIESI Farmaceutici SpA are the companies with the highest number of drugs in the approved phase, indicating their strong R&D progress. The most common indication for approved drugs targeting Cav2.1 is hypertension, highlighting the potential of these drugs in treating cardiovascular disorders. Small molecule drugs dominate the development phase, indicating intense competition around innovative drugs. China, the European Union, Japan, and the United States are the countries/locations with significant progress in the development of drugs targeting Cav2.1. Overall, the target Cav2.1 shows promising potential in the pharmaceutical industry, with multiple companies, indications, drug types, and countries/locations contributing to its development.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Nicardipine Hydrochloride is a small molecule drug developed by CHIESI Farmaceutici SpA. It targets Cav2.1 and is approved for the treatment of angina pectoris, hypertension, essential hypertension, and subarachnoid hemorrhage. Nicardipine Hydrochloride received its first approval in Japan in 1981 and subsequent approval in China. Due to its regulation as an orphan drug, it indicates its potential use in rare diseases or conditions.