Update on Phase III CAPItello-290 Study: Truqap and Chemotherapy for Advanced Triple-Negative Breast Cancer
The Phase III CAPItello-290 trial investigating Truqap (capivasertib) combined with paclitaxel in patients with either locally advanced or metastatic triple-negative breast cancer did not achieve its dual primary goals of enhancing overall survival when compared to the combination of paclitaxel and placebo. This outcome was observed both in the entire study population and in the subset of patients with tumors containing specific biomarker changes (PIK3CA, AKT1, or PTEN).
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Breast cancer ranks as the second most prevalent cancer globally and is a major cause of cancer-related fatalities. While certain breast cancers can be positive for estrogen receptors, progesterone receptors, or show overexpression of human epidermal growth factor receptor 2 (HER2), triple-negative breast cancer (TNBC) is characterized by the absence of all three markers.
In initial treatment phases, around 59,000 TNBC patients are typically treated with a specific medication. Mutations in PIK3CA and AKT1, along with PTEN modifications, impact about 35% of individuals with TNBC.
Dr. Peter Schmid, principal investigator at Barts Cancer Institute in London, UK, noted: "Even with some advances, treating triple-negative breast cancer continues to be very challenging due to the scarcity of actionable biomarker targets, keeping chemotherapy-based treatments as the standard approach."
Susan Galbraith, Executive Vice President of Oncology R&D at AstraZeneca, commented: “We are dedicated to advancing science for patients with some of the toughest cancers, including this diverse subtype of breast cancer. Although the CAPItello-290 results are disappointing, they will contribute to our understanding of the PI3K/AKT pathway in breast cancer as we carry on with our clinical trials in the Truqap development program and beyond.”
The safety profile of Truqap in combination with paclitaxel in the CAPItello-290 study aligned with the established safety profiles of the individual medicines, with no new safety concerns. The data will be disclosed in due time.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
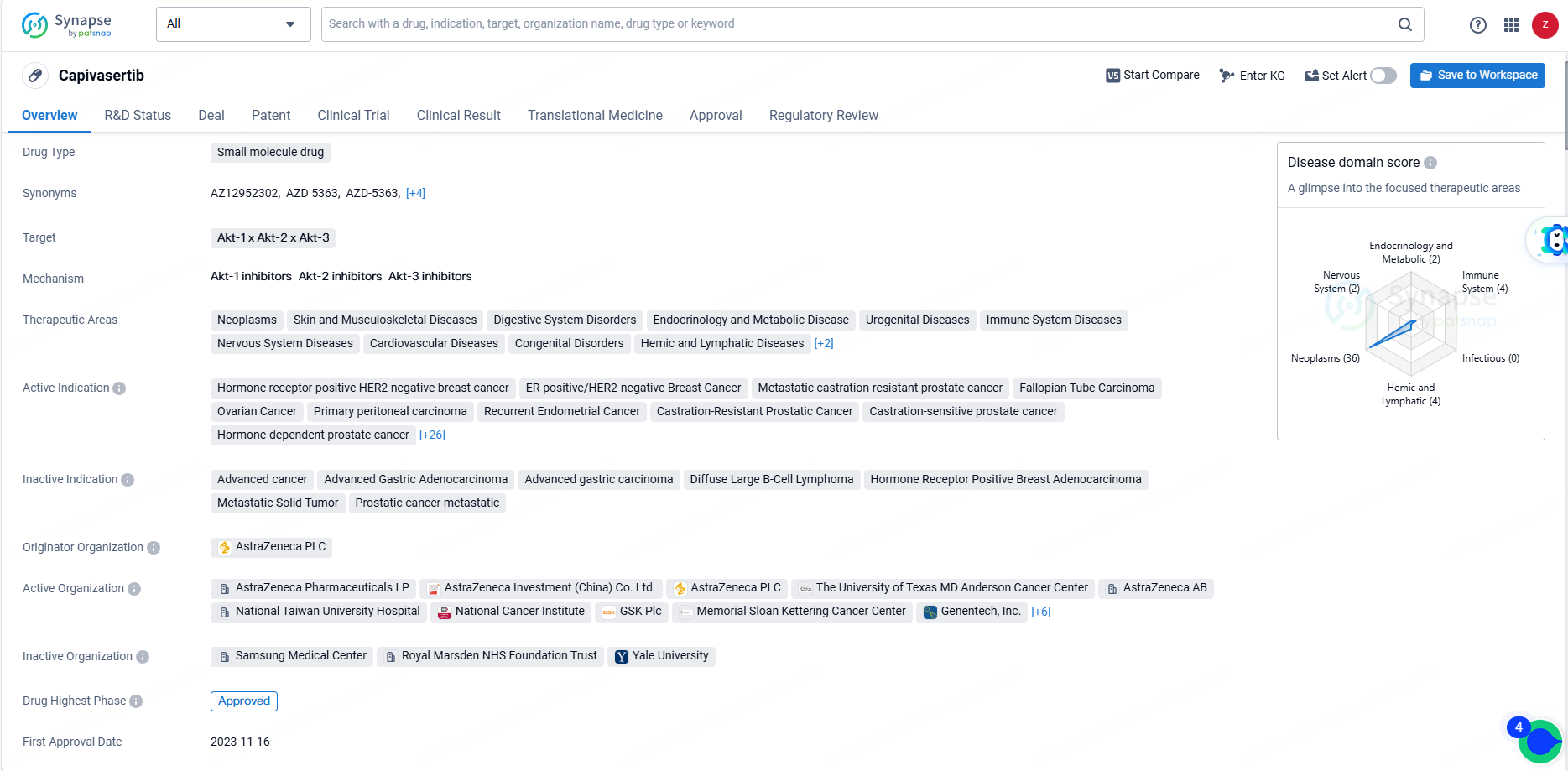
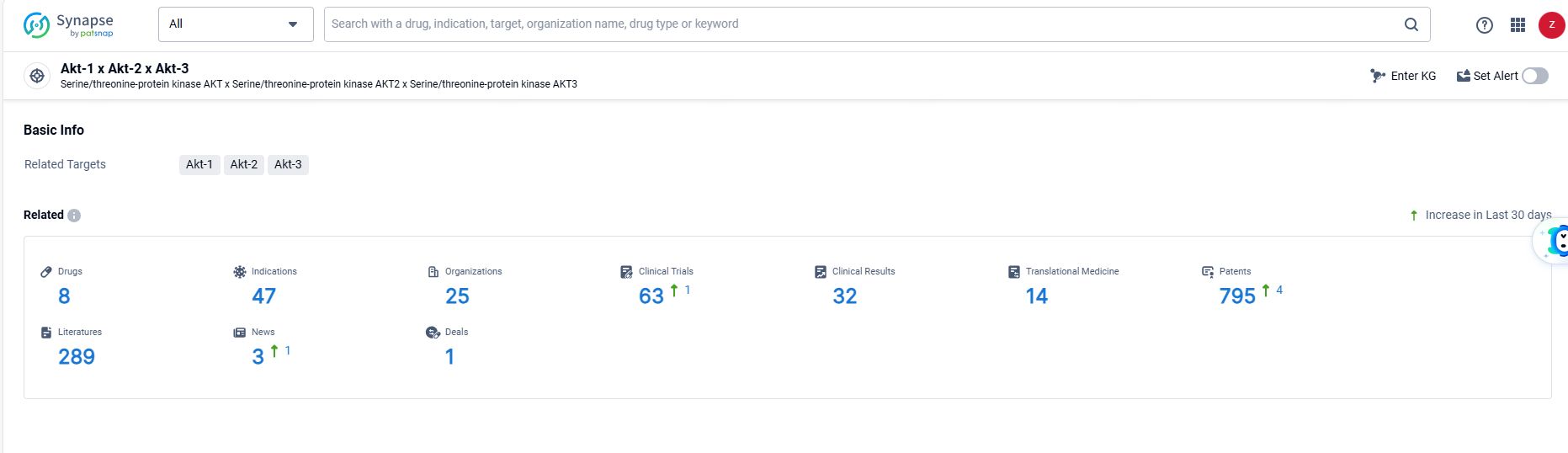
According to the data provided by the Synapse Database, As of June 21, 2024, there are 8 investigational drugs for the Akt-1, Akt-2, and Akt-3 target, including 47 indications, 25 R&D institutions involved, with related clinical trials reaching 63, and as many as 795 patents.
Capivasertib received its first approval in the United States in November 2023, with a priority review designation. It has also undergone the New Drug Application/Biologics License Application process in China. Capivasertib's approval marks a significant milestone in the pharmaceutical industry, offering a promising treatment option for a wide range of oncological and other related conditions.