Upstream Bio begins Phase II trial for Verekitug (UPB-101) in Chronic Rhinosinusitis with Nasal Polyps, treating first patients
Upstream Bio, a biopharmaceutical firm focused on developing innovative treatments for inflammatory conditions, disclosed the initiation of patient treatment in a Phase 2 study, examining the therapeutic potential of verekitug in individuals suffering from chronic rhinosinusitis accompanied by nasal polyps.
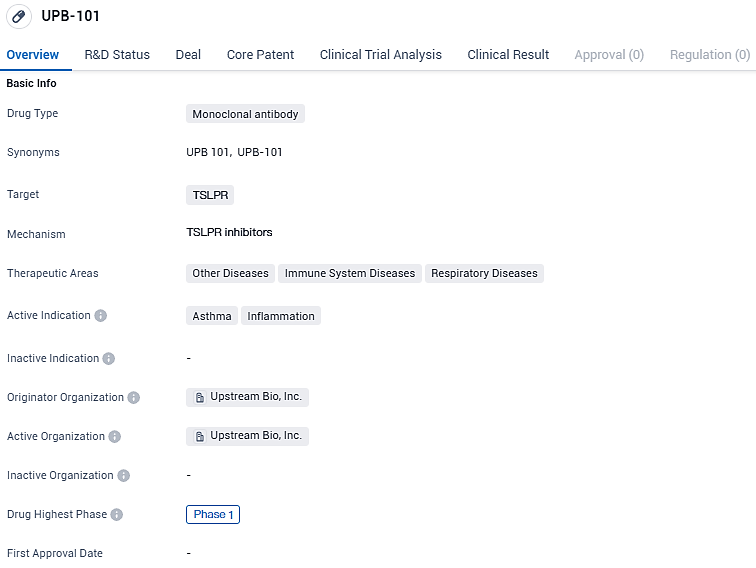
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Verekitug constitutes a synthetic, fully humanized IgG1 mAb developed to antagonize the thymic stromal lymphopoietin receptor (TLSPR), thereby curbing TSLP-mediated inflammatory processes. TSLP, an inflammatory cytokine, plays a pivotal role in the pathogenesis of CRSwNP, asthma, and several other allergic and inflammatory conditions.
"We are very excited to advance verekitug to a Phase 2 trial in CRSwNP, following the significant and persistent impact we observed on fractional exhaled nitric oxide (FeNO) and circulating eosinophil counts in the recently concluded Phase 1b trial in asthma subjects," remarked Aaron Deykin, MD, the lead of Clinical and Research Development. "The robust results from previous studies provide a foundation for administering verekitug at a minimum 12-week interval," he added.
The Phase 2 study for CRSwNP is a methodically structured, double-masked, placebo-controlled trial that will administer a 100 mg dose of verekitug via subcutaneous injection once every 12 weeks. The trial aims to determine the drug's effectiveness in managing CRSwNP, with the primary measure being the reduction in the severity of nasal polyps. Insights gained from combining the outcomes of Upstream Bio's Phase 2 endeavours in CRSwNP and asthma will shape the dosing regimen for the Phase 3 trials within both conditions.
"The present Phase 2 trial offers Upstream Bio an excellent prospect to showcase verekitug's capacity to serve as a superior biologic agent across a spectrum of allergic and inflammatory ailments," expressed Sam Truex, the company's CEO. "Anticipating that the profound changes in disease markers we detected in the Phase 1b will carry over to the Phase 2 study, we believe CRSwNP patients will significantly benefit from a regimen involving a single injection every 12 weeks."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
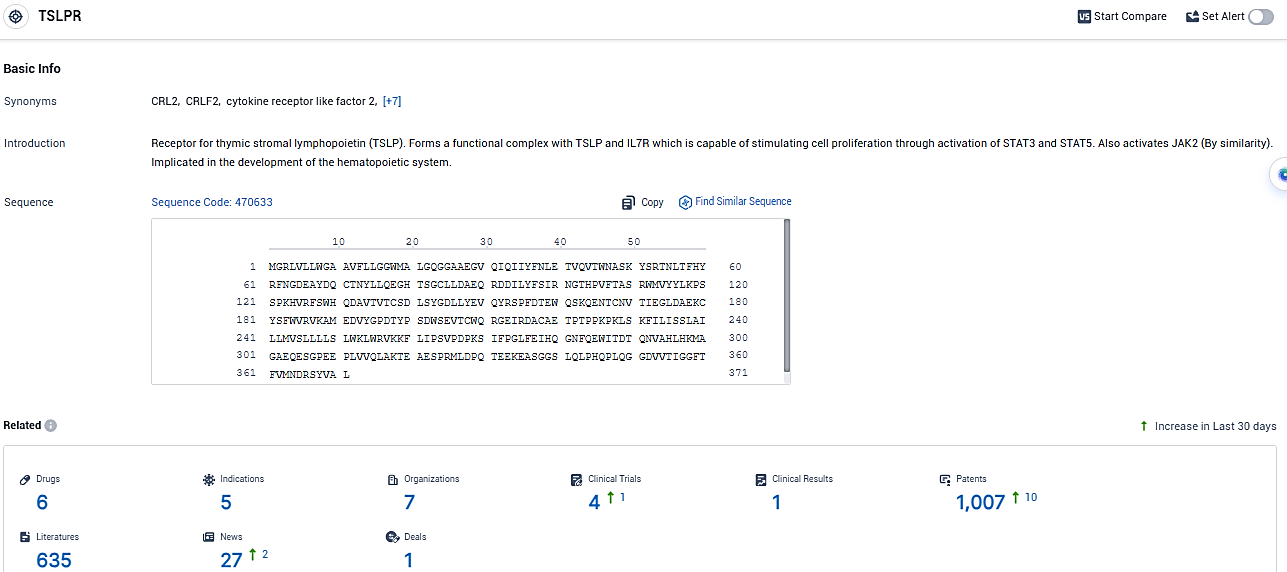 According to the data provided by the Synapse Database, As of January 6, 2024, there are 6 investigational drugs for the TSLPR target, including 5 indications, 7 R&D institutions involved, with related clinical trials reaching 4, and as many as 1007 patents.
According to the data provided by the Synapse Database, As of January 6, 2024, there are 6 investigational drugs for the TSLPR target, including 5 indications, 7 R&D institutions involved, with related clinical trials reaching 4, and as many as 1007 patents.
Verekitug is a novel recombinant fully human immunoglobulin G1 monoclonal antibody that binds to the human thymic stromal lymphopoietin receptor (TSLPR) to inhibit signaling.UPB-101 is being investigated for its potential in treating asthma and inflammation, as well as other diseases related to the immune system and respiratory system. As it is still in the early stages of development, further research and clinical trials will be needed to determine its safety and efficacy.