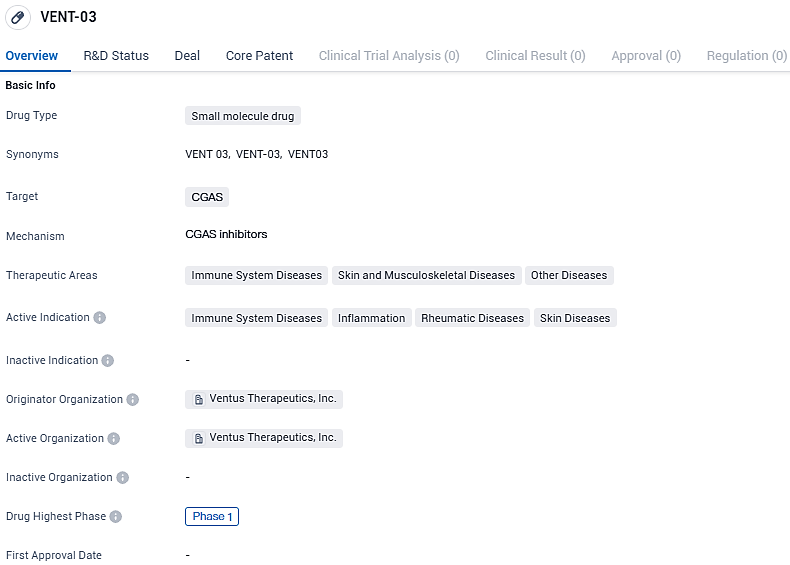
Ventus Therapeutics starts trials on VENT-03, a drug to inhibit cGAS, a novel strategy in its class
Ventus Therapeutics, an enterprise in the clinical phase of biopharmaceutical development with a focus on proprietary technology for structural biology and computational chemistry known as ReSOLVETM, today declared the initiation of a Phase 1 clinical study for VENT-03 by administering the initial dose to a subject. This marks a significant milestone as VENT-03 is the pioneer cGAS inhibitor to progress into the realm of clinical trials.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The pharmaceutical sector has long grappled with the challenge of creating an effective cGAS inhibitor, a task which to date, has proven quite challenging. Yet, we have taken a significant leap forward by initiating a Phase 1 clinical study," proclaimed Marcelo Bigal, M.D., Ph.D., who holds the dual role of President and CEO at Ventus. "Our team is fully committed to pushing this promising candidate forward, hoping to tackle the unaddressed needs of patients grappling with inflammatory conditions. With this trial, Ventus is maintaining its forward trajectory, marking this as the second project to enter clinical stages within a six-month time frame," Bigal further stated.
The inaugural human-based Phase 1 trial for VENT-03 is meticulously structured to delve into the drug's pharmacokinetic and pharmacodynamic profiles, along with its safety parameters, encompassing a comprehensive span of both singular and multiple dose escalations in a pool of healthy participants. Ventus anticipates unveiling the findings from this study in the latter portion of 2024.
Ventus' Chief Scientific Officer, Mike Crackower, Ph.D., emphasized the significance of initiating the VENT-03 Phase 1 study, remarking on the accomplishment of targeting a historically challenging molecule. "Our proprietary ReSOLVETM technology, which synergistically harnesses the strengths of machine learning, physics, and computational chemistry, provided pivotal insights into the cGAS binding site. These insights were instrumental in our development of VENT-03, positioning it as a pioneering drug in its class with impressive inhibitory strength."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
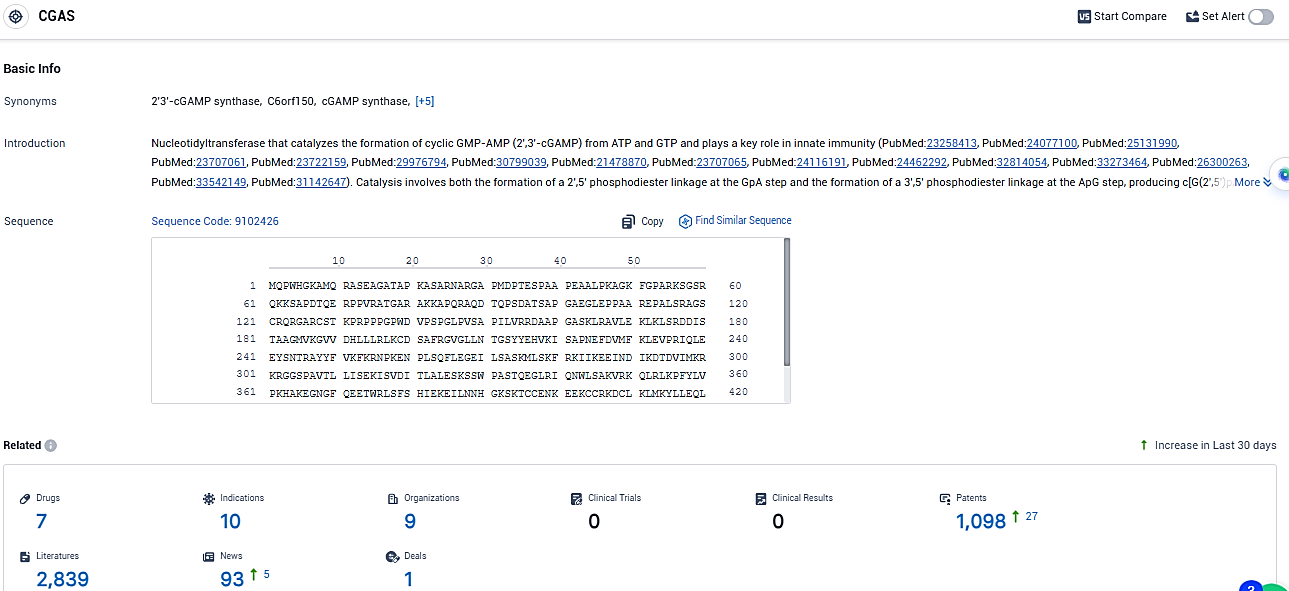
According to the data provided by the Synapse Database, As of January 6, 2024, there are 7 investigational drugs for the CGAS target, including 10 indications, 9 R&D institutions involved, and as many as 1098 patents.
VENT-03 targets CGAS and is being developed for the treatment of immune system diseases, skin and musculoskeletal diseases, and other diseases. The drug is currently in Phase 1 of clinical development, indicating its early stage of testing in humans. Further research and clinical trials will be needed to determine the drug's efficacy and safety profile in treating the indicated conditions.