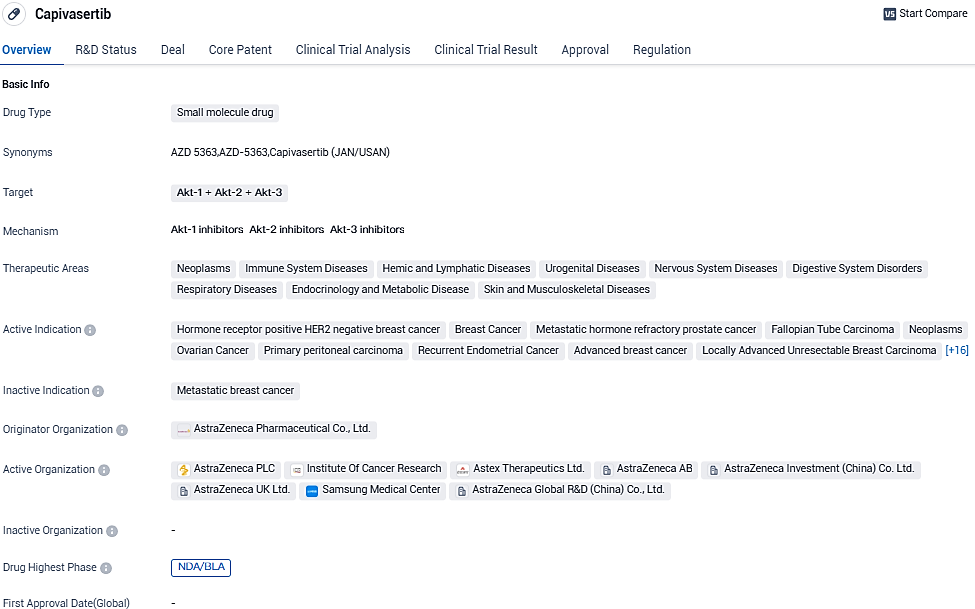
US approved Truqap (capivasertib) and Faslodex combo for advanced HR-positive breast cancer
In the United States, AstraZeneca's Truqap (capivasertib) paired with Faslodex (fulvestrant) has obtained approval for the therapy of HR-positive, HER2-negative advanced or metastatic breast cancer in adult patients with at least one biomarker alteration (PIK3CA, AKT1 or PTEN). These patients should have shown progression following a minimum of one endocrine-based treatment in a metastatic scenario or had a recurrence within or after 12 months of finishing adjuvant therapy.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The sanction by the FDA was decided upon the outcomes of the CAPItello-291 Phase III trial issued in The New England Journal of Medicine earlier this year. In this study, the amalgamation of Truqap and Faslodex shrunk the likelihood of tumour growth or mortality by 50% as compared to Faslodex by itself in individuals having tumours with PI3K/AKT pathway biomarker distortions.
The most prevalent form of cancer, breast cancer, is a leading source of cancer-related fatalities globally. Hormone Receptor (HR) positive breast cancer is the dominant subtype with over 65% of tumours identified as HR-positive with low HER2 levels or HER2-negative. Generally, alterations in PIK3CA and AKT1 along with deviations in PTEN transpire quite often, impacting up to half of the patients with metastatic HR-positive breast cancer.
The most frequently used therapy for this circumstance is endocrine therapy, however, a lot of patients become resilient to first-line treatments such as cyclin-dependent kinase 4/6 inhibitors and estrogen receptor-focussed therapies. This emphasizes the urgency for more options based on endocrine therapy.
Medical Oncologist at Memorial Sloan Kettering Cancer Center, Komal Jhaveri, MD, stated: “There's a desperate need to augment the potency of first-line endocrine therapies that have an extensive usage among patients suffering from metastatic HR-positive breast cancer as tumour development or resilience is an eminent issue. A blend of capivasertib and fulvestrant, a unique combination, brings forth an extremely needed new approach for treating roughly half of the patients having these marked biomarkers in this scenario, potentially preventing disease growth.”
Dave Fredrickson, Executive Vice President, Oncology Business Unit, AstraZeneca, commented: “This sanction of a first-class medicine opens up an essential new selection for US patients who have this distinct illness and we eagerly wait to launch Truqap for the large number of global breast cancer patients who can benefit from it.”
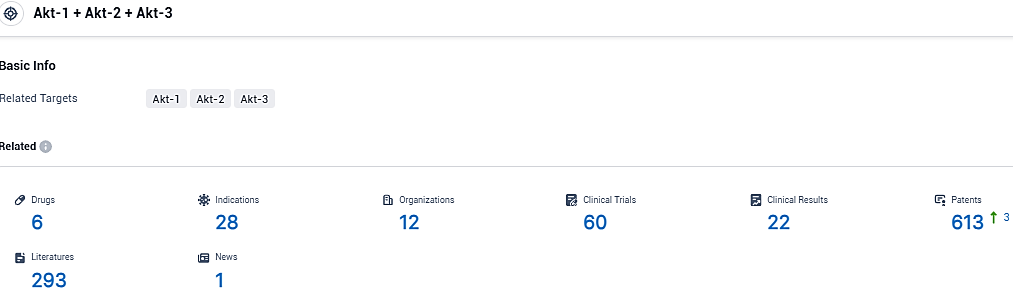
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 23, 2023, there are 6 investigational drugs for the Akt-1 and Akt-2 and Akt-3 target, including 28 indications, 12 R&D institutions involved, with related clinical trials reaching 60, and as many as 613 patents.
Truqap (capivasertib) is a first-in-class, potent, ATP-competitive inhibitor of all three AKT isoforms (AKT1/2/3). The US regulatory submission was granted Priority Review and reviewed under Project Orbis, which provides a framework for concurrent submission and review of oncology medicines among participating international partners. As part of Project Orbis, Truqap plus Faslodex is also under review by regulatory authorities in Australia, Brazil, Canada, Israel, Singapore, Switzerland and the UK.