US Approves TAGRISSO® and Chemotherapy Combo for Advanced EGFR-Mutated Lung Cancer
The US has granted authorization for AstraZeneca’s TAGRISSO® (osimertinib) when used alongside chemotherapy in the care of mature individuals suffering from non-small cell lung cancer that is either at an advanced local stage or has metastasized and exhibits mutations in the epidermal growth factor.
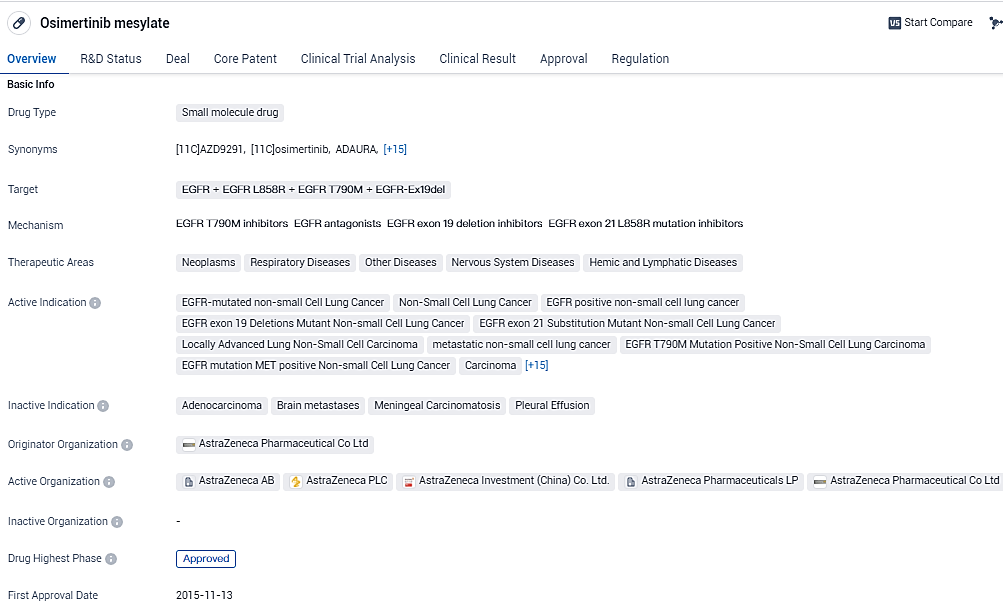
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The Food and Drug Administration granted authorization following an expedited Priority Review, drawing from the findings of the FLAURA2 Phase III clinical study, as detailed in The New England Journal of Medicine. Combination therapy involving TAGRISSO and chemotherapy led to a 38% decrease in the risk of disease progression or death when compared to the use of TAGRISSO alone, which sets the primary standard for global care.
Investigator-assessed median progression-free survival (PFS) extended to 25.5 months for those receiving the combined treatment of TAGRISSO and chemotherapy, marking an increase of 8.8 months over the use of TAGRISSO by itself.
This extension in survival was confirmed through a separate evaluation by a blinded independent central review, recording a median PFS of 29.4 months for patients on the combined regimen, outperforming the solo treatment by 9.5 months.
Annually, over 200,000 Americans are diagnosed with lung cancer. Of these cases, 80-85% are classified as non-small cell lung cancer (NSCLC), making it the predominant type. Advanced stages of NSCLC are identified in approximately 70% of these individuals. Also, an EGFR mutation is present in about 15% of US NSCLC patients.
Dr. Pasi A. Jänne, a Dana-Farber Cancer Institute medical oncologist and lead researcher for the FLAURA2 trial, remarked that the newly sanctioned treatment option, made viable by FLAURA2's exceptional data, offers a substantial advancement for those with advanced, EGFR-mutant NSCLC. Physicians now have access to a pair of potent osimertinib-based treatments, allowing for more customized therapeutic approaches aimed at optimizing patient outcomes.
In December 2023, the combination of TAGRISSO and chemotherapy got incorporated into the NCCN Clinical Practice Guidelines in Oncology as a Category 1 Other Recommended protocol for managing NSCLC patients with certain EGFR mutations, underpinned by data from the FLAURA2 trial.
TAGRISSO has received approval for use as a single agent in upwards of 100 nations, including the US, EU, China, and Japan. Its sanctioned uses encompass first-line treatment for patients with locally advanced or metastatic EGFRm NSCLC, local or metastatic NSCLC harboring the EGFR T790M mutation, and as an adjuvant treatment of early-stage EGFRm NSCLC. Further regulatory reviews in various countries are ongoing, anchored by the FLAURA2 findings.
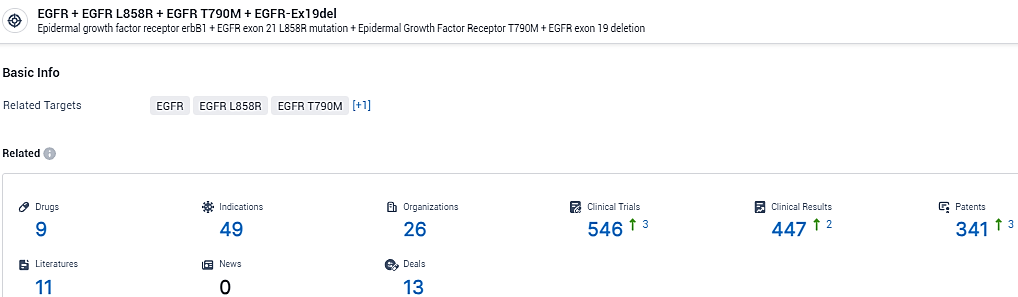
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 23, 2024, there are 9 investigational drugs for the EGFR and EGFR L858R and EGFR T790M and EGFR-Ex19del target, including 49 indications,26 R&D institutions involved, with related clinical trials reaching 546, and as many as 341 patents.
TAGRISSO® (osimertinib) is a third-generation, irreversible EGFR-TKI with proven clinical activity in NSCLC, including against CNS metastases. Its approval in multiple countries and involvement in various regulatory processes highlight its importance in the field of biomedicine.