U.S. Greenlights Early Review of New Treatment for Advanced Non-Squamous NSCLC
The U.S. regulatory authorities have approved the review of Daiichi Sankyo and AstraZeneca's request for authorization – known as a Biologics License Application – for their drug, datopotamab deruxtecan (Dato-DXd). This review process is a significant step in making the treatment available for adults diagnosed with a form of lung cancer that is either at a later, localized stage or has spread (known as non-squamous non-small cell lung cancer) and who have previously undergone systemic treatment.
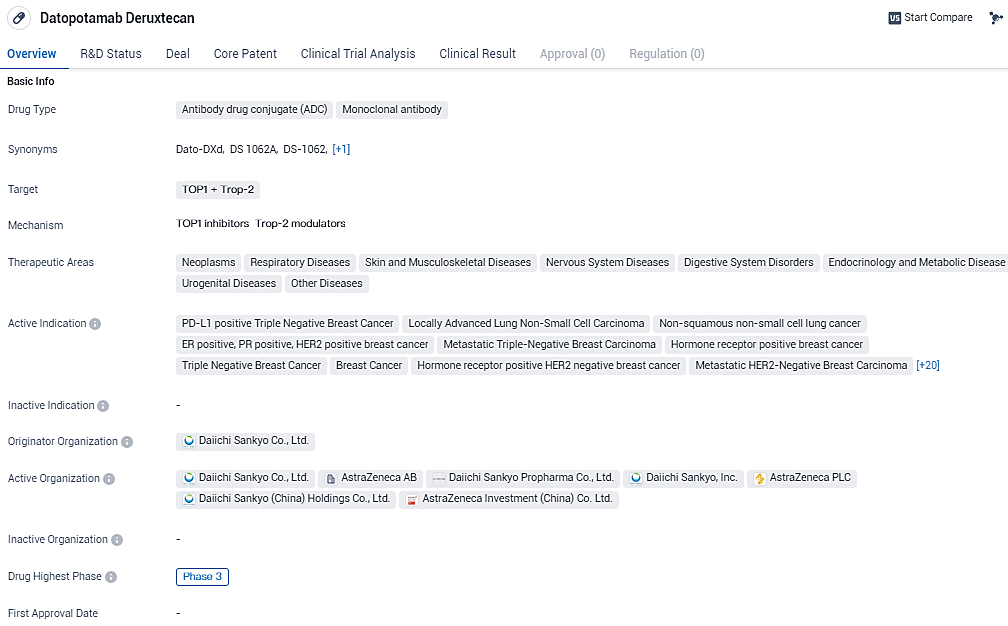
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Datopotamab deruxtecan, a precisely created antibody drug conjugate targeting TROP2, is under co-development by Daiichi Sankyo and AstraZeneca. The Prescription Drug User Fee Act (PDUFA) goal date for a decision by the U.S. Food and Drug Administration is set for December 20, 2024.
The BLA submission leverages data from the TROPION-Lung01 phase 3 trial, which was highlighted at the Presidential Symposium during the 2023 European Society for Medical Oncology Congress. In this crucial study, datopotamab deruxtecan significantly prolonged progression-free survival, a major dual endpoint, when compared with docetaxel—previously established as standard care—in patients with advanced or spread NSCLC who had undergone at least one earlier treatment.
While interim findings pointed to datopotamab deruxtecan potentially improving overall survival more than docetaxel in the main study group, statistical significance wasn't achieved by the cut-off date. Nonetheless, for those with nonsquamous NSCLC, datopotamab deruxtecan presented a substantial advantage in progression-free survival and suggested a positive impact on overall survival.
The research continues, with the final overall survival analysis still pending. The safety findings for datopotamab deruxtecan align with those from similar ongoing studies, without any new risks surfacing.
Additionally, a separate BLA is under consideration in the U.S. for datopotamab deruxtecan, relying on findings from the TROPION-Breast01 phase 3 study. This application targets the treatment of adults with metastatic hormone receptor-positive, HER2-negative breast cancer. Worldwide, other regulatory filings to approve datopotamab deruxtecan for lung and breast cancer treatments are also in progress.
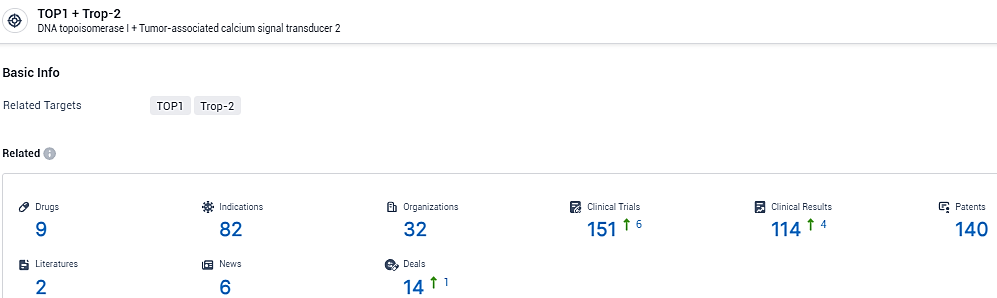
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 23, 2024, there are 9 investigational drugs for the TOP1 and Trop-2 target, including 82 indications, 32 R&D institutions involved, with related clinical trials reaching 151, and as many as 140 patents.
Datopotamab deruxtecan appears to be a promising drug with a wide range of potential therapeutic applications. Its targeting of TOP1 and Trop-2 suggests its potential effectiveness in treating various types of cancers, including breast, lung, prostate, colorectal, and ovarian cancers. The drug's advancement to phase 3 trials indicates that it has shown promising results in earlier stages of development.