U.S. FDA Approves Takeda's ENTYVIO (vedolizumab) for continued treatment of severe active Ulcerative Colitis via under-the-skin injection
Takeda communicated that the U.S. FDA has given their approval for a subcutaneous delivery of ENTYVIO®(vedolizumab) to serve as a maintenance treatment for adults suffering from moderately to severely active ulcerative colitis, following an induction therapy using ENTYVIO administered intravenously.
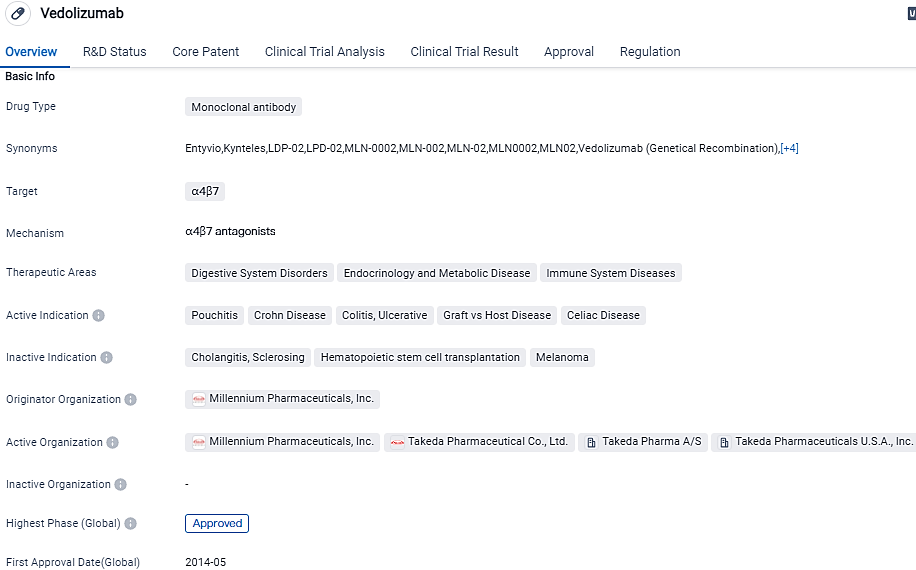
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The availability of ENTYVIO SC in the U.S. as a single-dose prepackaged pen is projected for the conclusion of October. Furthermore, a Biologics License Application for an exploratory SC dispensation of ENTYVIO to treat adults suffering from moderately to severely active Crohn’s disease is currently under the FDA's scrutiny.
Following the FDA's endorsement of subcutaneous ENTYVIO, patients and doctors hoping for ENTYVIO's clinical profile coupled with adaptable administration now have dual options for maintenance care for adults with moderate to high severity ulcerative colitis", explicated Brandon Monk, senior vice president, chief, U.S. Gastroenterology Business Unit, Takeda.
Expanding further, Brandon Monk added "Takeda is dedicated to answer the diverse healthcare requirements, situations, and personal preferences of individuals afflicted with UC as they tread through their enduring ordeal with the affliction. ENTYVIO stands as the solitary FDA-accredited biologic for upkeep treatment in ulcerative colitis, providing the alternative for either intravenous or subcutaneous dispersion”.
Vedolizumab serves as a biologic therapy and holds approval for intravenous and subcutaneous administration. Vedolizumab SC has earned marketing authorization in the United States, the European Union, and beyond 50 countries. Vedolizumab IV has acquired marketing authorization in over 70 countries, with the including the United States and the European Union. Worldwide, Vedolizumab IV and SC boast over a million patient years of exposure thus far.
Vedolizumab is a distinctively designed humanized monoclonal antibody aiming to counter the alpha4beta7 integrin, thus restraining the combination of alpha4beta7 integrin with intestinal mucosal addressin cell adhesion molecule 1, but not impeding the vascular cell adhesion molecule 1. MAdCAM-1 majorly shows up on blood vessels and lymph nodes of the gastrointestinal system. Through the inhibition of alpha4beta7 integrin, vedolizumab likely restricts the capacity of particular white blood cells to penetrate gut tissues.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 5, 2023, there are 24 investigational drugs for the α4β7 target, including 15 indications,31 R&D institutions involved, with related clinical trials reaching 215,and as many as 2518 patents.
Vedolizumab is a considerable breakthrough in biomedicine, providing specialized treatment solutions for diverse digestive disorders, endocrinological and metabolic illnesses, as well as immune diseases. It has received acceptance in many countries and therapy fields, emphasizing its ability to tackle unresolved health requirements.