Vertex Presents Promising Long-Term Results for CASGEVY™ at 2024 EHA Congress
Vertex Pharmaceuticals Incorporated has presented extended-term results for CASGEVY™ (exagamglogene autotemcel [exa-cel]) derived from international clinical studies involving patients with severe sickle cell disease or transfusion-dependent beta thalassemia. These findings, shared at the European Hematology Association's annual conference, reinforce the transformative, reliable, and long-lasting clinical advantages of CASGEVY. CASGEVY remains the only authorized CRISPR-based gene-editing treatment available.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The data being shared come from over 100 patients treated with exa-cel in clinical trials, with the longest follow-up period now surpassing 5 years. The efficacy outcomes align with the previously reported primary and significant secondary endpoints analyses from these exa-cel studies, and they continue to show transformative clinical benefits with sustained and stable levels of fetal hemoglobin and allelic editing.
"The substantial benefit seen in patients with sickle cell disease during the trial is remarkable, given the severe and ongoing burden of disease that individuals with this blood disorder endure," remarked Haydar Frangoul, M.D., M.S., Medical Director of Pediatric Hematology and Oncology at Sarah Cannon Research Institute and HCA Healthcare’s TriStar Centennial Children’s Hospital. "I look forward to offering this therapy and the prospect of a potential functional cure to my eligible patients."
"The extensive data presented today for adult and adolescent TDT patients reinforce the growing evidence base for CASGEVY, and it is crucial now to ensure that this innovative treatment becomes available to patients in the real-world setting as soon as possible," stated Franco Locatelli, M.D., Ph.D., Professor of Pediatrics at the Catholic University of the Sacred Heart in Rome and Director of the Department of Pediatric Hematology and Oncology at Bambino Gesù Children’s Hospital.
In the long-term follow-up study CLIMB-131, two out of the three patients who did not reach TI12 in CLIMB-111 achieved TI12 and have remained transfusion-independent for over a year. The third patient has been free from transfusions for 3.4 months.
CASGEVY™ is a non-viral, ex vivo CRISPR/Cas9 gene-edited cell therapy designed for eligible patients with SCD or TDT. It involves editing a patient’s own hematopoietic stem and progenitor cells at the erythroid-specific enhancer region of the BCL11A gene through a precise double-strand break. This editing leads to the generation of high levels of fetal hemoglobin (HbF; hemoglobin F) in the red blood cells. HbF is the form of hemoglobin that carries oxygen during fetal development, which transitions to the adult form of hemoglobin after birth.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
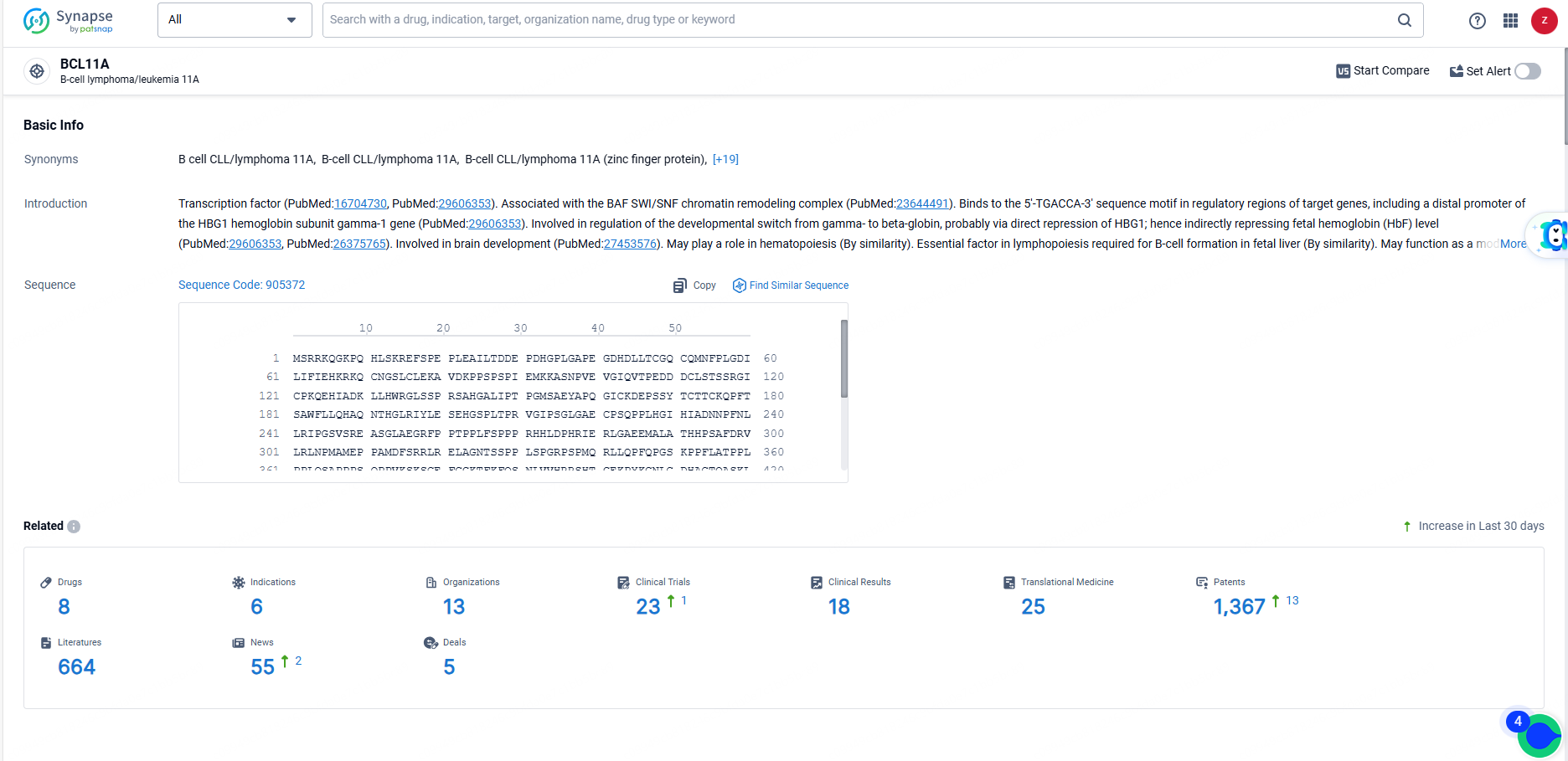
According to the data provided by the Synapse Database, As of June 18, 2024, there are 8 investigational drugs for the BCL11A target, including 6 indications, 13 R&D institutions involved, with related clinical trials reaching 23, and as many as 1367 patents.
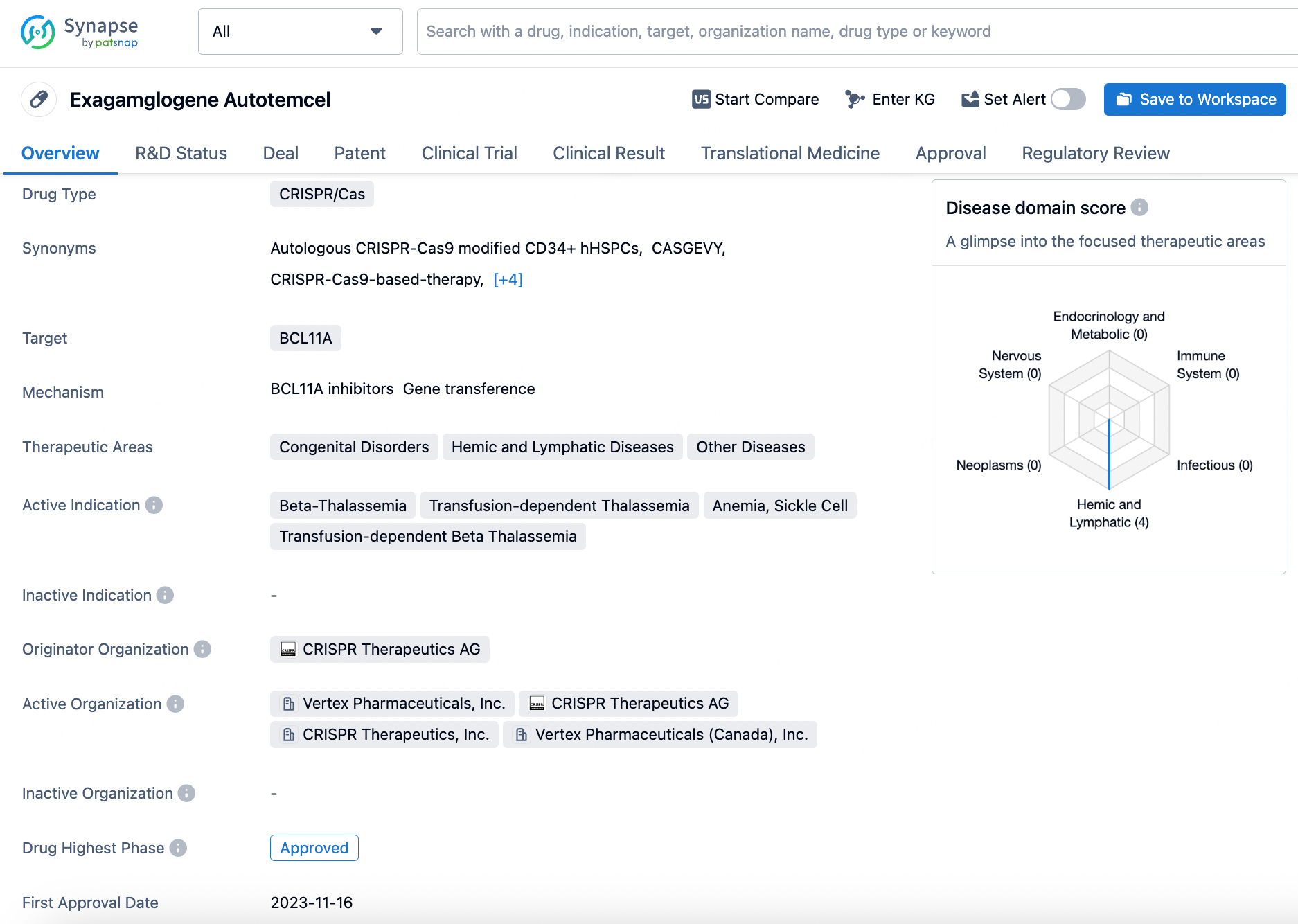
Exagamglogene Autotemcel represents a significant advancement in the treatment of various blood disorders, particularly those related to thalassemia and anemia. Its approval in the United Kingdom and the various regulatory designations it has received indicate its potential to address unmet medical needs and provide therapeutic benefits to patients.