Zura Bio Unveils Findings for Tibulizumab (ZB-106) Initiative at EULAR 2024
Zura Bio Limited, an immunology company in the clinical-stage focused on creating innovative dual-pathway antibodies for autoimmune and inflammatory conditions, has recently unveiled positive results from a Phase 1 trial of its leading drug candidate, tibulizumab (ZB-106), aimed at treating Sjogren’s syndrome. Additionally, preclinical findings endorsing the continued development of tibulizumab for rheumatoid arthritis were also disclosed at the Annual European Congress of Rheumatology 2024 held in Vienna.
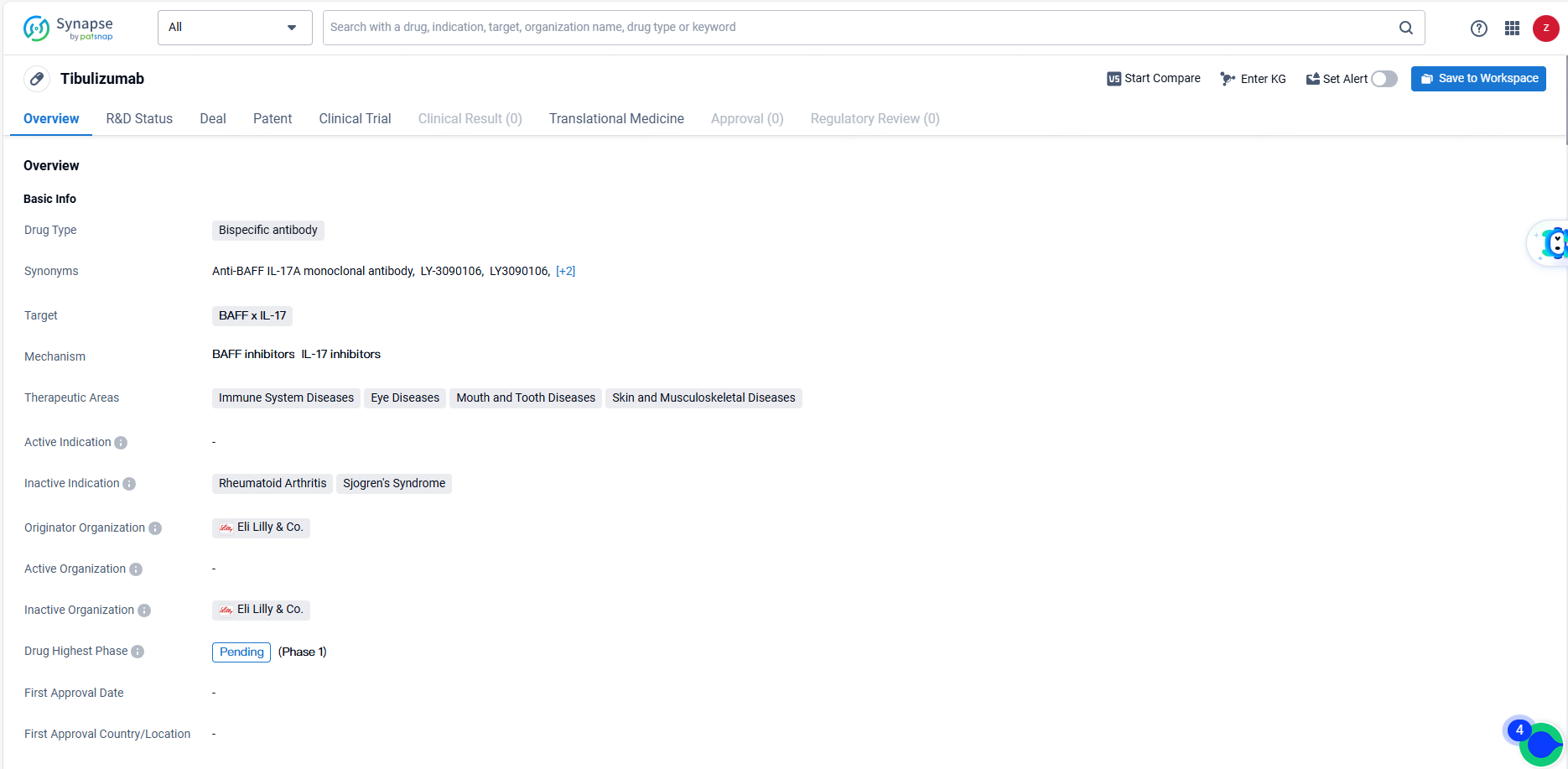
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"Collectively, this dataset contributes to early-phase evidence showing that simultaneously inhibiting IL-17A and BAFF might be a pioneering strategy for autoimmune and inflammatory disorders, where inhibition of a single pathway is currently standard practice," remarked Robert Lisicki, Chief Executive Officer.
"In Sjogren’s syndrome, the findings illustrate that tibulizumab effectively achieved significant target engagement, reaching near-maximum serum concentrations after single, well-tolerated subcutaneous doses administered at four-week intervals. Additionally, preclinical outcomes suggest that dual-pathway inhibition should be clinically investigated in RA and other autoimmune disorders, expanding the therapeutic potential of tibulizumab," added Lisicki.
Following tibulizumab administration, serum levels of total IL-17A and BAFF increased, indicating successful target engagement. At dosages of 100 mg Q4W and above, the concentrations of these markers plateaued, implying that near-maximal target engagement was achieved.
Throughout the study, there was a dose-dependent decrease in total B cell counts among all participants, and tibulizumab administration was linked with reduced levels of Th1 cells. Tibulizumab also modulated inflammatory mediators such as serum amyloid A, interleukins 5 and 10, as well as basic fibroblast growth factor. These findings suggest tibulizumab holds therapeutic promise for a range of additional autoimmune conditions.
Tibulizumab, a humanized bispecific dual antagonist antibody, fuses TALTZ® (ixekizumab) with tabalumab, engineered to bind and neutralize IL-17A and BAFF. Tibulizumab is slated to enter Phase 2 clinical trials for systemic sclerosis in Q4 2024 and hidradenitis suppurativa in Q2 2025. Completed tibulizumab studies include Phase 1/1b trials in Sjogren's syndrome and rheumatoid arthritis.
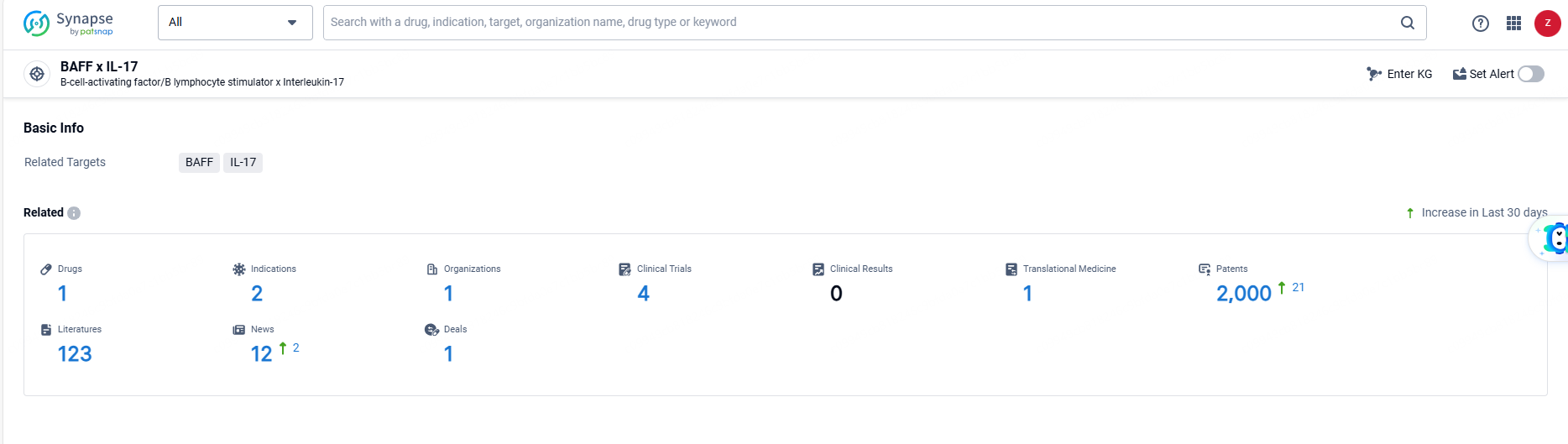
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 18, 2024, there are 1 investigational drugs for the IL-17A and BAFF targets, including 2 indications, 1 R&D institutions involved, with related clinical trials reaching 4, and as many as 2000 patents.
Tibulizumab targets BAFF and IL-17 for the treatment of immune system diseases, eye diseases, mouth and tooth diseases, as well as skin and musculoskeletal diseases. Its potential to address multiple disease pathways simultaneously makes it a promising candidate for various conditions, pending further development and regulatory approval.