Vincerx Announces Positive Preliminary Results from VIP943 Phase 1 Study and Updates on Pipeline and Corporate Progress
Vincerx Pharma, Inc. (Nasdaq: VINC), a company in the biopharmaceutical sector focusing on transformative therapies for unaddressed cancer patient needs, has reported two complete responses in its initial human trial. This Phase 1 study involves dose escalation of VIP943, the company's advanced antibody-drug conjugate (ADC), which is under investigation for treatment in cases of relapsed or refractory acute myeloid leukemia (AML), higher-risk myelodysplastic syndrome (HR-MDS), and B-cell acute lymphoblastic leukemia (B-ALL). Furthermore, the company shared updates on its drug pipeline and corporate developments.
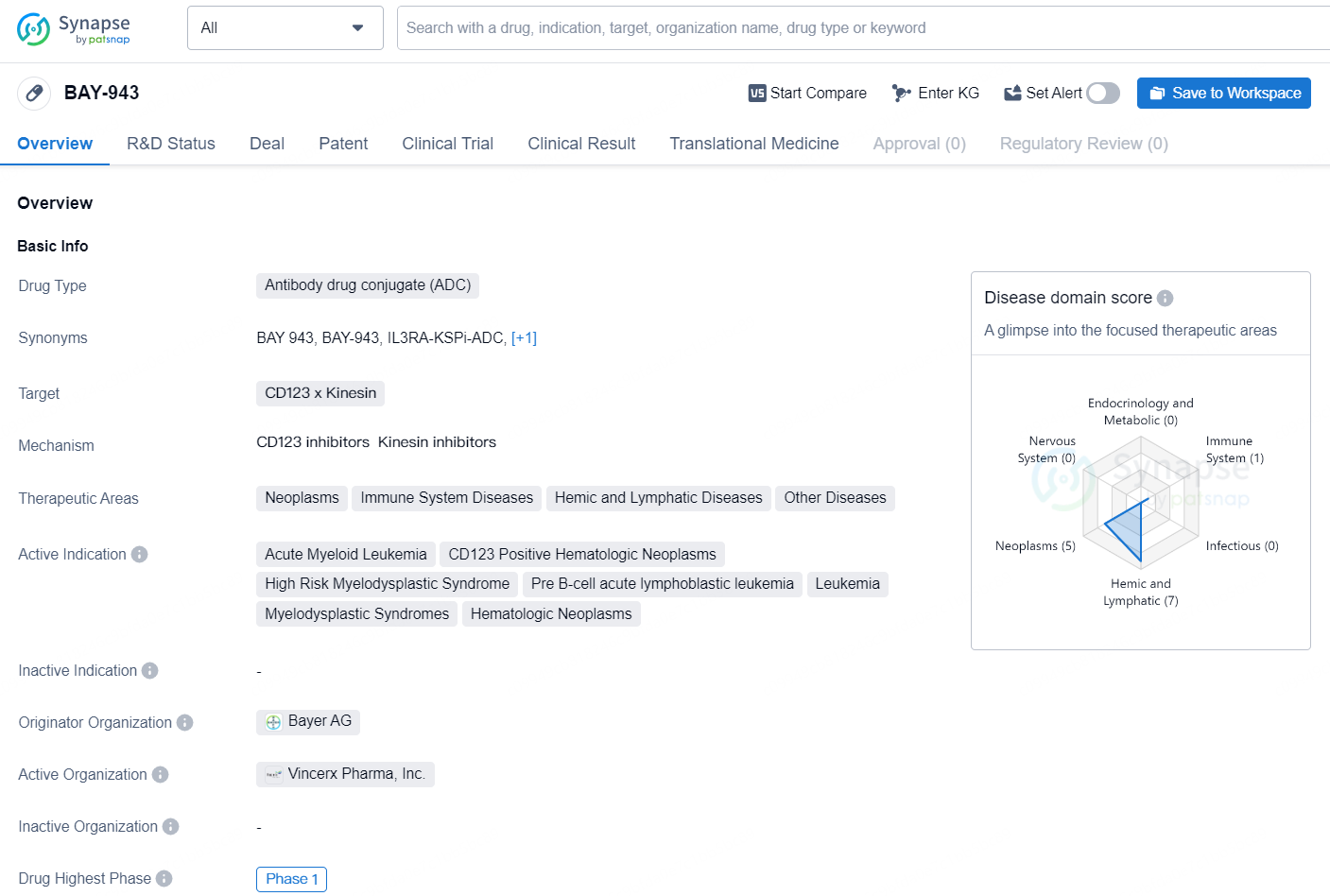
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The current Phase 1 study exploring the dose escalation of VIP943 has, to date, included 22 participants across different increasing dose groups ranging from 0.2 to 1.3 mg/kg administered weekly. These participants are part of a challenging-to-manage salvage patient group that typically does not respond well to single-drug treatments. Out of these 22, nine individuals (comprising six with AML and three with HR-MDS) have been administered a minimum of three doses at an effective level of VIP943 (i.e., ≥1.0 mg/kg). Among these nine, four (44%) continue to be part of the study. Thus far, one patient suffering from relapsed AML has reached complete remission with incomplete hematologic improvement (CRi), and another patient with HR-MDS has reached complete remission with limited count recovery (CRL), judged by internationally accepted response criteria. These benchmarks are well-established as viable evaluation criteria in AML and MDS research, emphasizing the importance of these initial findings.
"We're encouraged by the new data from our Phase 1 research involving VIP943, which shows clinical efficacy in patients that are difficult to treat," stated Ahmed Hamdy, M.D., the CEO of Vincerx. "We think these encouraging clinical outcomes underscore VIP943's potential as a leading therapy for CD123+ blood cancers and confirm our VersAptx platform's capability to produce safer, more effective bioconjugates by solving challenges faced by traditional ADCs."
As of August 2024, VIP943 has displayed a positive safety profile and is well-tolerated, with no dose-limiting toxicities noted among the 22 participants. Serious adverse events (SAEs) align with the expectations for this type of patient group. The most frequent SAEs included pneumonia (three participants, 14%), and both cellulitis and febrile neutropenia (two participants each, 9%). Only one participant (5%) encountered a drug-related SAE (Grade 3 diarrhea).
Evidence of target engagement, exemplified by receptor occupancy, has been confirmed by the binding of VIP943 to CD123+ peripheral blood blasts in AML patients involved in the Phase 1 study. The highest group dosage (1.3 mg/kg) achieved maximal receptor occupancy of 84%. Across all dosage groups, receptor occupancy was maintained for under 96 hours. Additionally, reductions in CD123+ peripheral blood blasts were seen following dosing.
These pharmacodynamic (PD) indicators reveal that VIP943 is effectively binding to and removing CD123+ cancerous cells. Initial pharmacokinetic (PK) results consistently show a minimal release of payload (≤1% in plasma). The half-life of VIP943 is under 96 hours, and no accumulation occurs with repeat administration. These PK and PD findings have prompted investigations into twice-weekly dosing of VIP943 as a possible "induction" strategy. Enrollment continues for both the weekly and twice-weekly dosage groups.
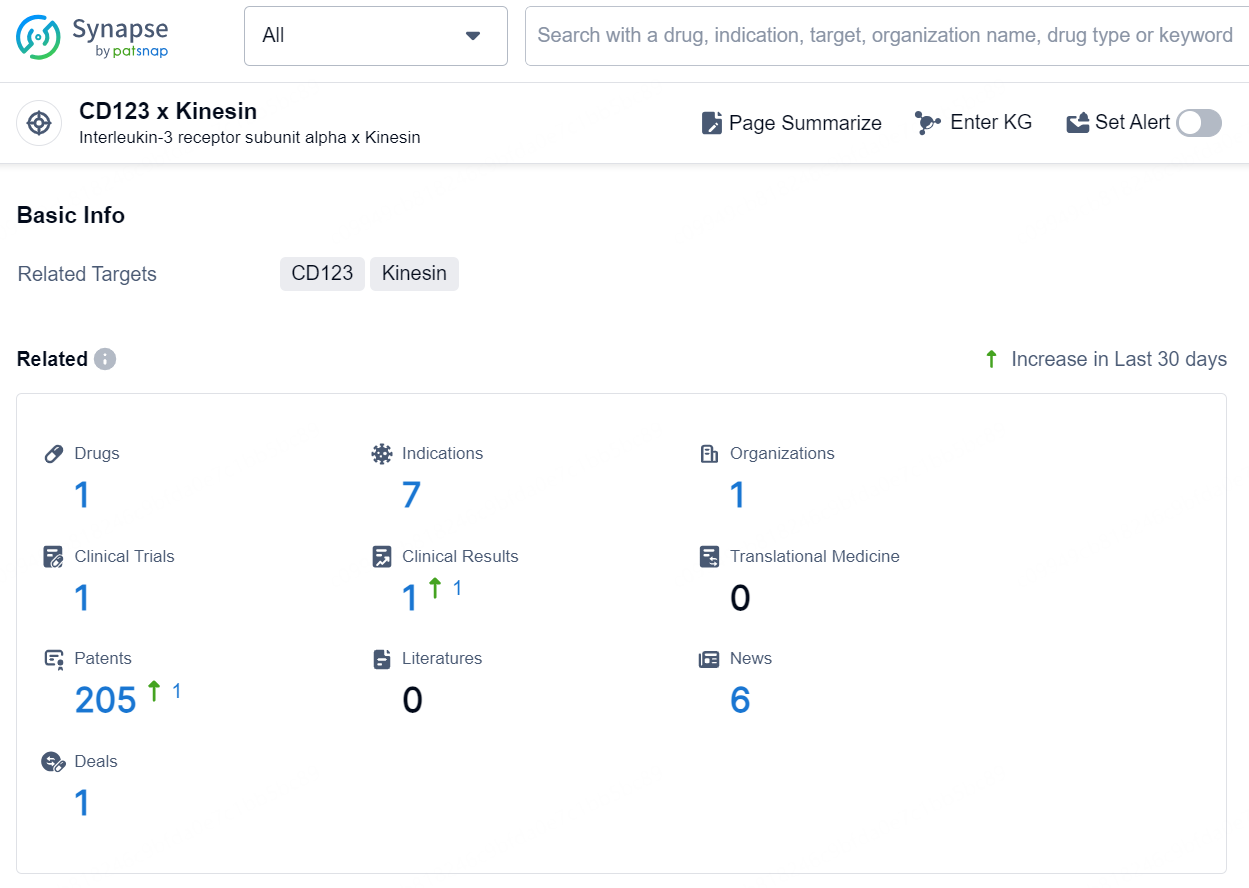
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of October 10, 2024, there are 1 investigational drug for the CD123 x Kinesin targets, including 7 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 205 patents.
The drug BAY-943 is classified as an Antibody Drug Conjugate (ADC) and targets CD123 x Kinesin. Its therapeutic areas include Neoplasms, Immune System Diseases, Hemic and Lymphatic Diseases, and Other Diseases. The drug is indicated for the treatment of various conditions such as Acute Myeloid Leukemia, CD123 Positive Hematologic Neoplasms, High Risk Myelodysplastic Syndrome, Pre B-cell acute lymphoblastic leukemia, Leukemia, Myelodysplastic Syndromes, and Hematologic Neoplasms.