Vittoria Biotherapeutics Announces FDA Clearance for VIPER-101 IND in T-Cell Lymphoma
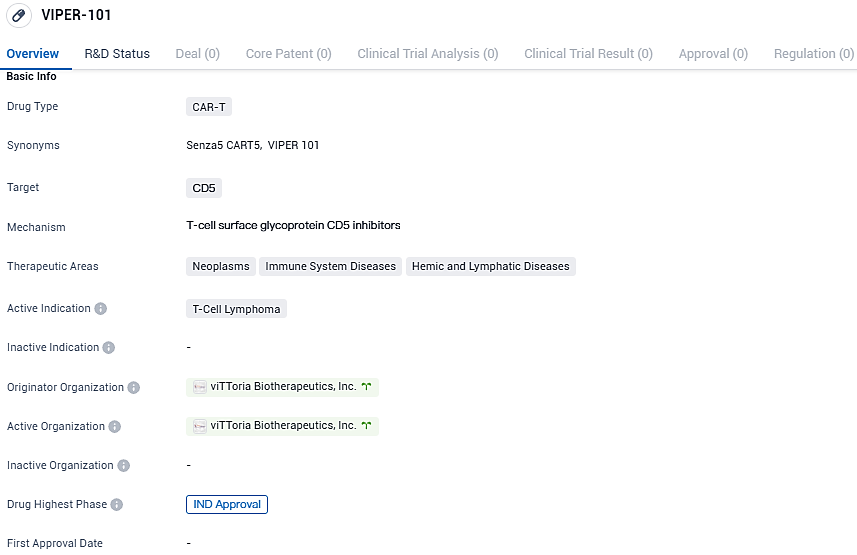
Vittoria Biotherapeutics has disclosed the approval of their Investigational New Drug (IND) submission by the FDA, paving the way for the commencement of a pioneering Phase 1 clinical trial in humans. This trial aims to assess the efficacy of VIPER-101, the leading therapeutic candidate from the company, which consists of a gene-edited, autologous CAR-T cell therapy, designed specifically for the treatment of individuals suffering from T-cell lymphoma that has either resisted previous treatment or recurred.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The authorization by the FDA of our experimental drug proposal for VIPER-101 signifies a significant breakthrough for our company, Vittoria Biotherapeutics, in our quest to improve treatment outcomes for those suffering from hard-to-treat illnesses,” stated Dr. Nicholas Siciliano, Ph.D., the CEO of Vittoria.
“Considering the few developments in the area of T-cell lymphoma therapies over the past years, this represents an essential progression in introducing a novel therapeutic approach to individuals with T-cell lymphoma, potentially revolutionizing their treatment results. This is made possible through our exclusive technology platform, Senza5, developed to augment effectiveness and enhance safety.” Dr. Siciliano elaborated further.
VIPER-101 is a personalized CAR-T therapy targeting CD5, designed for T-cell lymphoma, and is developed on Vittoria’s unique cell therapy engineering and production platform, Senza5TM. While CD5 serves as a general marker for T-cells and plays a role in an immune-downregulating pathway, conventional CAR-T treatments directed at CD5 for malignant T-cell conditions may induce self-targeting immune responses, reducing the treatment's potency.
VIPER-101 is innovatively constructed to prevent such self-targeting and to fully leverage the advantage of bypassing the suppressive CD5 pathway. Using a specialized five-day refining technique to maintain cellular youthfulness, VIPER-101's attributes are harmonized to optimize power, safety, and production process efficiency.
At the forthcoming ASH Annual Meeting in 2023, a series of abstracts are slated to be presented that display the significant preclinical outcomes obtained with VIPER-101, and that highlight the Senza5 platform's extensive promise and increased tumor-fighting capabilities in various cancer models.
Dr. Siciliano declared: “Our team’s intense commitment, which has been instrumental in our swift and cost-effective shift from the research stage to clinical phase, will remain our cornerstone as we usher VIPER-101 into clinical trials, with the start of phase 1 slated for the start of 2024.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
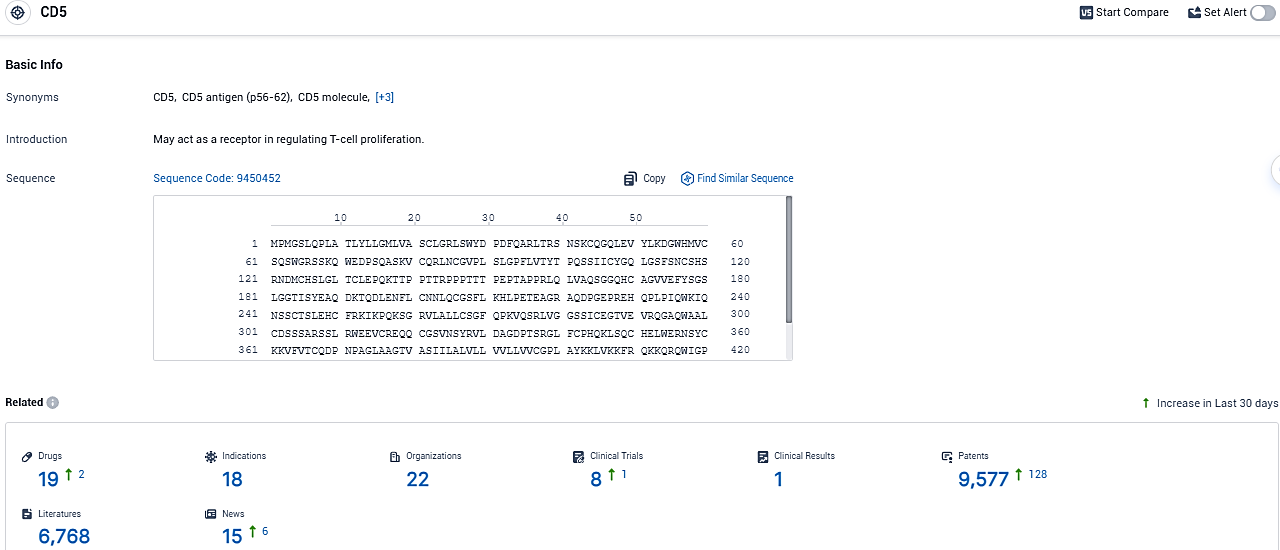
According to the data provided by the Synapse Database, As of December 15, 2023, there are 19 investigational drugs for the CD5 target, including 18 indications, 22 R&D institutions involved, with related clinical trials reaching 8, and as many as 9577 patents.
VIPER-101 targets CD5 and is intended for the treatment of T-cell lymphoma. The drug has received IND approval, indicating its potential as a therapeutic option for patients with this specific type of cancer.