Monopar Begins Phase 1 Trial of MNPR-101-Zr Radiotherapy for Advanced Cancer
Monopar Therapeutics Inc., a company in the clinical development phase that specializes in creating new therapies for individuals with cancer, has officially declared that the Human Research Ethics Committee in Australia has granted approval to initiate a Phase 1 dosimetry study for their unique radiopharmaceutical product, MNPR-101-Zr.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The inaugural clinical study of MNPR-101-Zr for Phase 1 dosimetry will be conducted among individuals diagnosed with progressive tumor conditions. It will leverage the capabilities of positron emission tomography (PET) to evaluate the absorption by tumors, the distribution within healthy tissue, and the overall secure application.
MNPR-101-Zr represents an isotope-labeled variant of Monopar's unique humanized monoclonal antibody, MNPR-101, designed with high specificity for the urokinase plasminogen activator receptor (uPAR).
Studies utilizing PET imaging in experimental animal models bearing cancers like triple-negative breast, colorectal, and pancreatic have shown that MNPR-101-Zr is taken up preferentially and substantially by tumors that express uPAR. These imaging findings concur with the outcomes from active in vivo studies that tested the efficacy of actinium-225 conjugated with MNPR-101 against preclinical xenograft cancer models, leading to further exploration of MNPR-101 as a precise radiopharmaceutical therapeutic for a range of severe oncological conditions.
The CEO of Monopar, Dr. Chandler Robinson, has acknowledged this development as a crucial checkpoint for the company, remarking, "With a development phase spanning over one and a half years that included rigorous studies, our positioning is strategic in the therapeutic market. This marks our premiere trial in humans with our innovative uPAR-targeted agent. Considering the robust clinical outcomes recently seen in the radiopharmaceutical field, particularly with agents targeting PSMA and SSTR2 in cancer, our expectations remain high for ushering in a new era in cancer therapy."
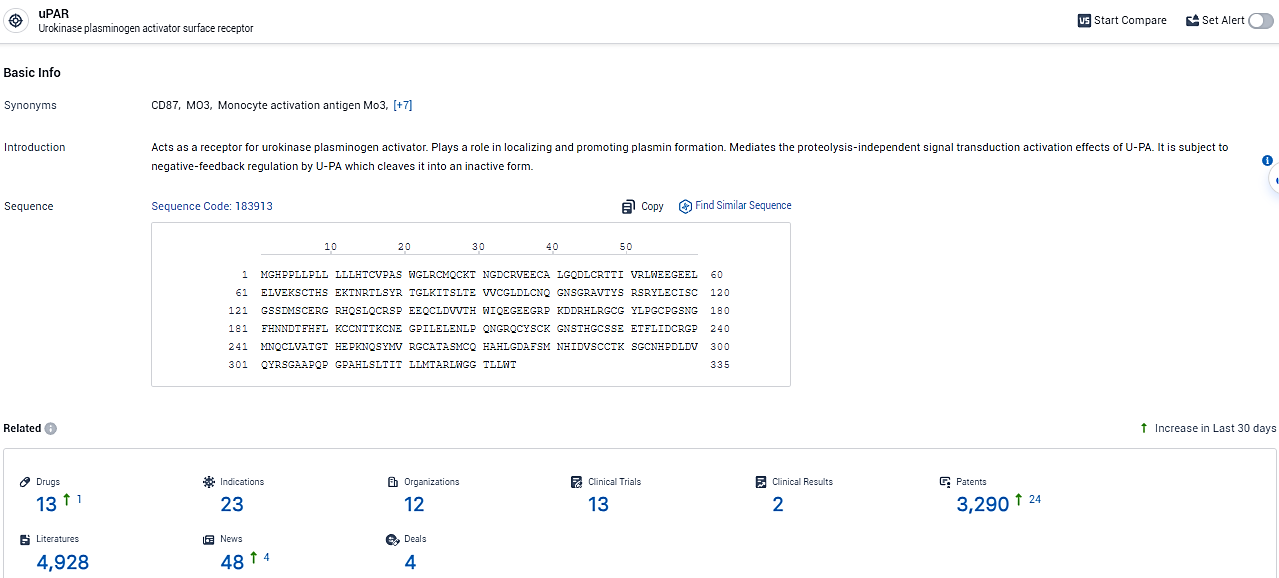
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 26, 2024, there are 13 investigational drugs for the uPAR target, including 23 indications,12 R&D institutions involved, with related clinical trials reaching 13, and as many as 3290 patents.
MNPR-101-Zr89 is a therapeutic radiopharmaceutical that targets uPAR and is being developed for the treatment of neoplasms. It is currently in the preclinical phase and is being developed by NorthStar Medical Radioisotopes LLC and Monopar Therapeutics, Inc. Further research and clinical trials will be necessary to determine the drug's potential as a treatment for cancer.