Ways to Evaluate the Worldwide R&D Progress of Our Study Sequence
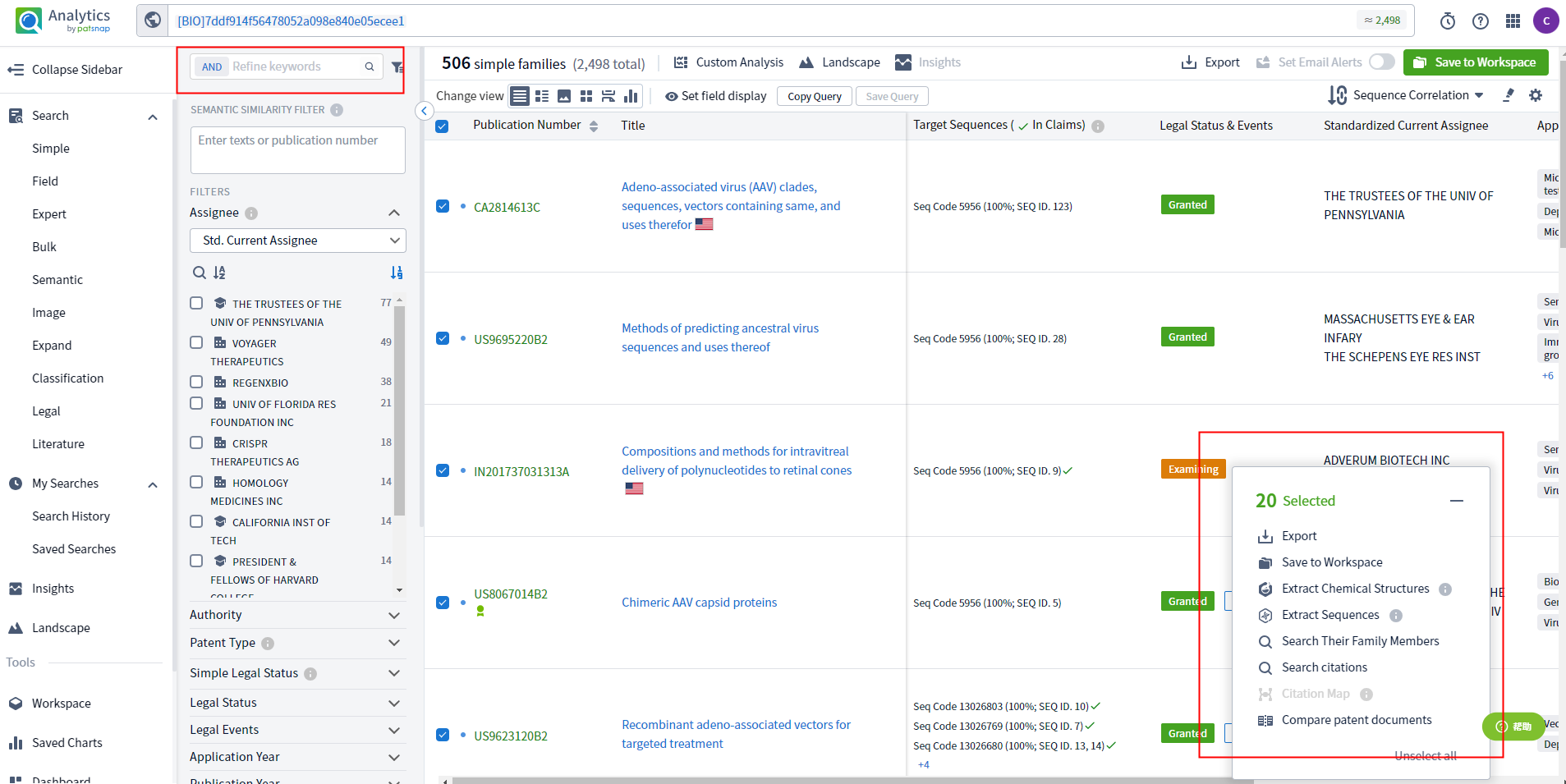
You can utilize databases for querying and analysis, and I believe the Patsnap Bio Sequence Database can be helpful. First, apply for a free account registration. In the "Sequence Search" section on the left side, enter your sequence using formats such as uploaded sequence, Sequence Code, GenBank ID, or CAS RN. Then, on the right side, select the sequence type and use advanced settings to specify parameters such as sequence similarity and length for the BLAST search. Click on the search button to present all the available data related to your sequence, including patents, biological journals, literature, and public biological databases.
This extensive collection of relevant data allows you to filter and refine the results based on various sequence selection options to focus on the specific data of interest. You can download mutation reports, export FASTA files, view public sources, save sequences, and set email alerts. You can access detailed information about the sequence for further research and analysis by clicking on patents, literature, journals, or other databases.
The Patsnap Bio Sequence Database offers comprehensive and convenient retrieval, allowing for fast searching and analysis of globally known similar sequences. It facilitates online sequence alignment and analysis, enabling you to understand risks and competitive intelligence better. Apply for an account and start exploring and sharing with your colleagues.
Free registration is currently available to utilize the Bio biological sequence database: https://bio-patsnap-com.libproxy1.nus.edu.sg. Act now to expedite your sequence search tasks.