What ADC-related progress will RemeGen reveal at the ASCO conference?
At this conference, RemeGen's antibody-drug conjugates (ADCs) including Disitamab Vedotin and the mesothelin-targeting ADC RC88, will present 16 latest clinical research data across two innovative drugs, showcasing in 1 clinical science discussion, 5 poster presentations, and 10 online abstracts, and covering multiple cancer types such as stomach cancer, bladder cancer, and gynecological tumors.
Disitamab Vedotin (RC48)
Disitamab Vedotin (RC48) has been used for the treatment of gastric and urothelial carcinomas and received conditional approval in China in June and December of 2021 respectively. In August 2021, Seagen (now acquired by Pfizer) secured an exclusive global license (excluding Asia-Pacific) for Disitamab Vedotin in a deal worth up to $2.6 billion, which includes a $200 million upfront payment and up to $2.4 billion in milestone payments, along with royalties ranging from high single-digit percentages to above fifteen percent.
The indications discussed at this meeting primarily focus on the effectiveness in recurrent or metastatic cervical cancer and muscle-invasive bladder cancer. According to data disclosed by the company at the CSCO Gynecological Cancer Annual Meeting in April this year, a Phase II, open-label, multi-center basket trial evaluated the efficacy and safety of RC48 monotherapy in HER2-expressing recurrent cervical cancer. In this trial, the RC48 dose was 2 mg/kg (Q2W). Among the 22 evaluable patients, the median progression-free survival (mPFS) was 4.37 months, the median duration of response (mDoR) was 5.52 months, and the one-year overall survival (OS) rate was 66%, particularly with a primary study endpoint objective response rate (ORR) reaching 36.4%. Subgroup analysis showed significant benefit in patients with HER2 IHC 1+ (ORR rate 50%), and RC48 also benefited those previously treated with anti-PD1, paclitaxel, and platinum therapy, showing impressive therapeutic efficacy in second-line and later treatments for recurrent cervical cancer. As for safety, the incidence of grade ≥3 treatment-related adverse events (TRAEs) was low, including decreased neutrophil count (12%), increased γ-glutamyl transferase (8%), and elevated alanine transaminase (8%). The overall safety profile was tolerable and controllable, with no ocular, pulmonary toxicity, or fatal TRAEs observed.
Furthermore, for bladder cancer indications, based on previously disclosed data from the RC48-C005/C009 studies, Disitamab Vedotin in patients with previously failed chemotherapy for bladder cancer consistently achieves an objective response rate of 50.5%, with a median progression time of 5.9 months and a median survival time of 14.2 months, effectively extending patient survival.
RC88
RC88 is a mesothelin-targeted ADC (Antibody-Drug Conjugate) developed in-house by RemeGen. It is currently in Phase II clinical trials. RC88 utilizes RemeGen's innovative bridging technology for antibody-drug linkage, featuring a structure that includes a mesothelin-targeting antibody, a cleavable linker, and the small molecule cytotoxin MMAE (monomethyl auristatin E). This design allows for targeted binding to MSLN-positive tumor cells, facilitating the internalization of the antibody and effectively delivering the cytotoxin directly to cancer cells, thereby achieving substantial tumor-killing effects. The drug employs a disulfide bridge linker that offers a uniform Drug-to-Antibody Ratio (DAR) and maintains stable antibody protein structure, leading to higher efficacy and improved safety.
In March 2023, an application for a Phase I/II clinical study of RC88 in combination with toripalimab for the treatment of patients with advanced malignant solid tumors was approved by China's CDE. In December 2023, an application for a Phase II clinical trial of RC88 in the treatment of gynecological tumors received approval from the U.S. FDA, and international multicenter clinical studies are set to commence across the United States, China, the European Union, and other regions. In January 2024, the FDA granted Fast Track Designation (FTD) to this drug for the treatment of patients with platinum-resistant recurrent epithelial ovarian cancer, fallopian tube cancer, and primary peritoneal cancer (PROC).
How to search for and analyze the development progress of ADC pharmaceuticals?
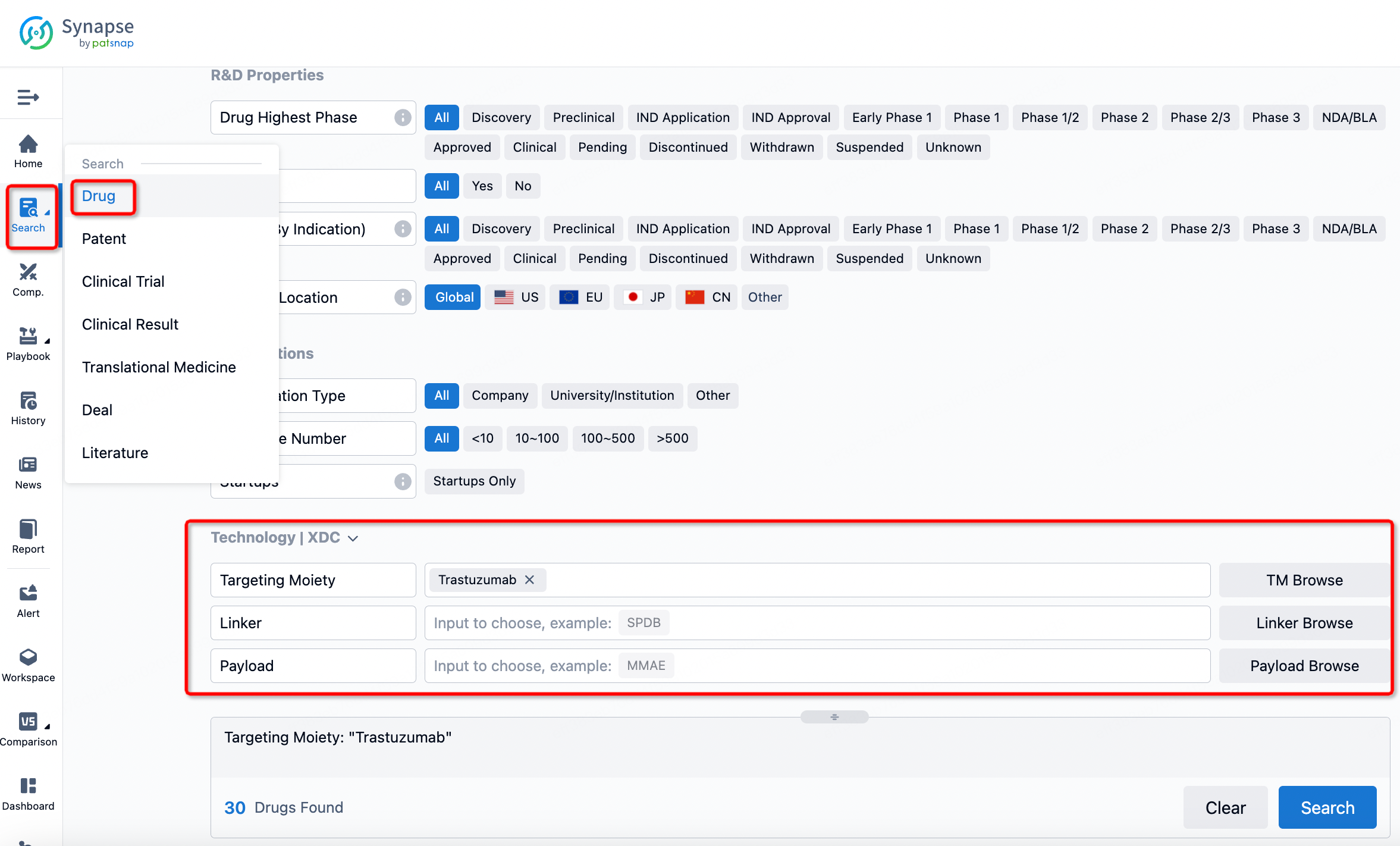
If you want to learn about the latest developments in ADC drugs, you can use the drug search module of the Synapse database. This module supports searching for ADC drugs by classification through Targeting Moiety, Linker, and Payload.
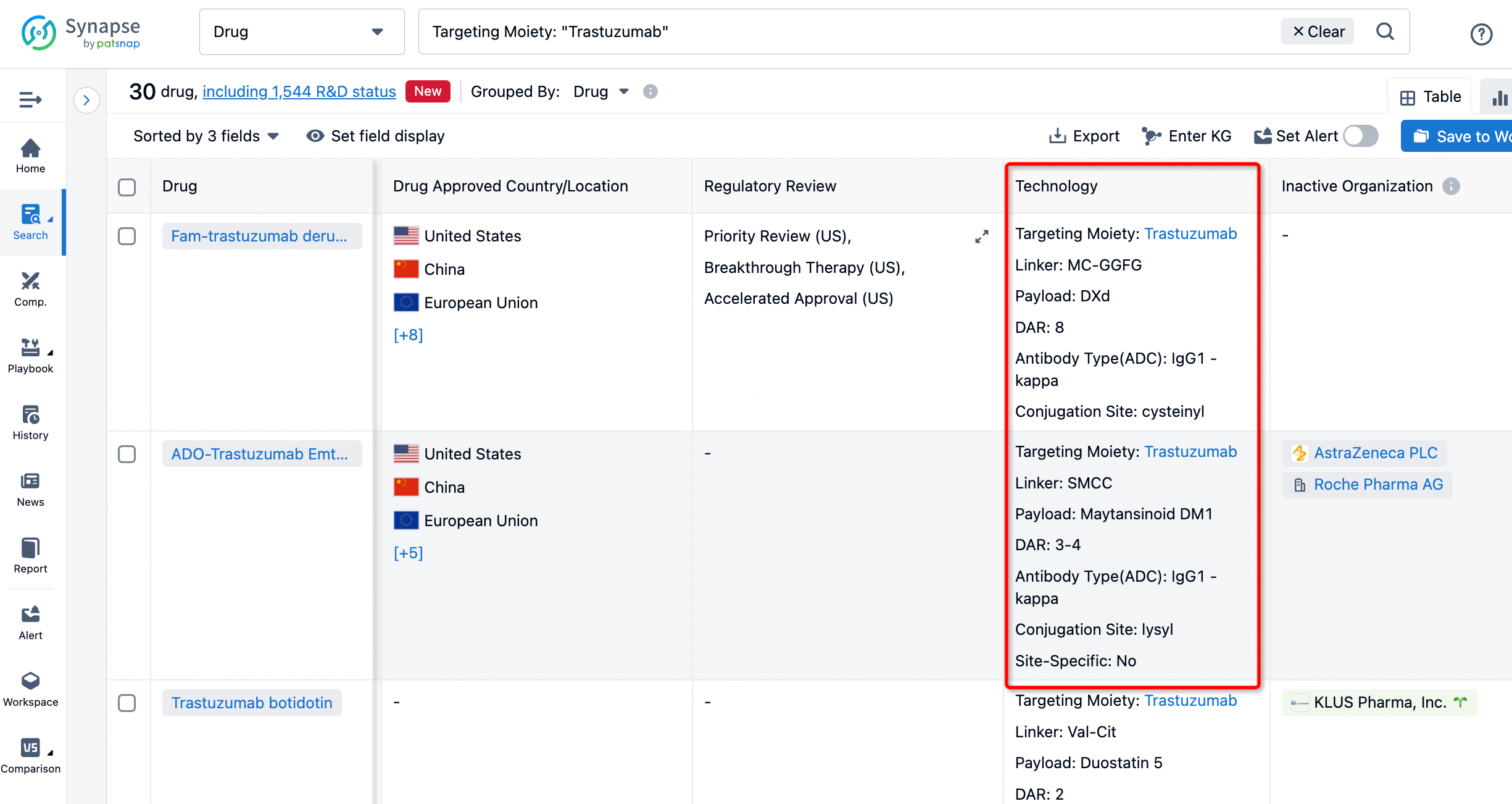
On the search results page, you can easily review information related to the ADC's technical category of the drugs.
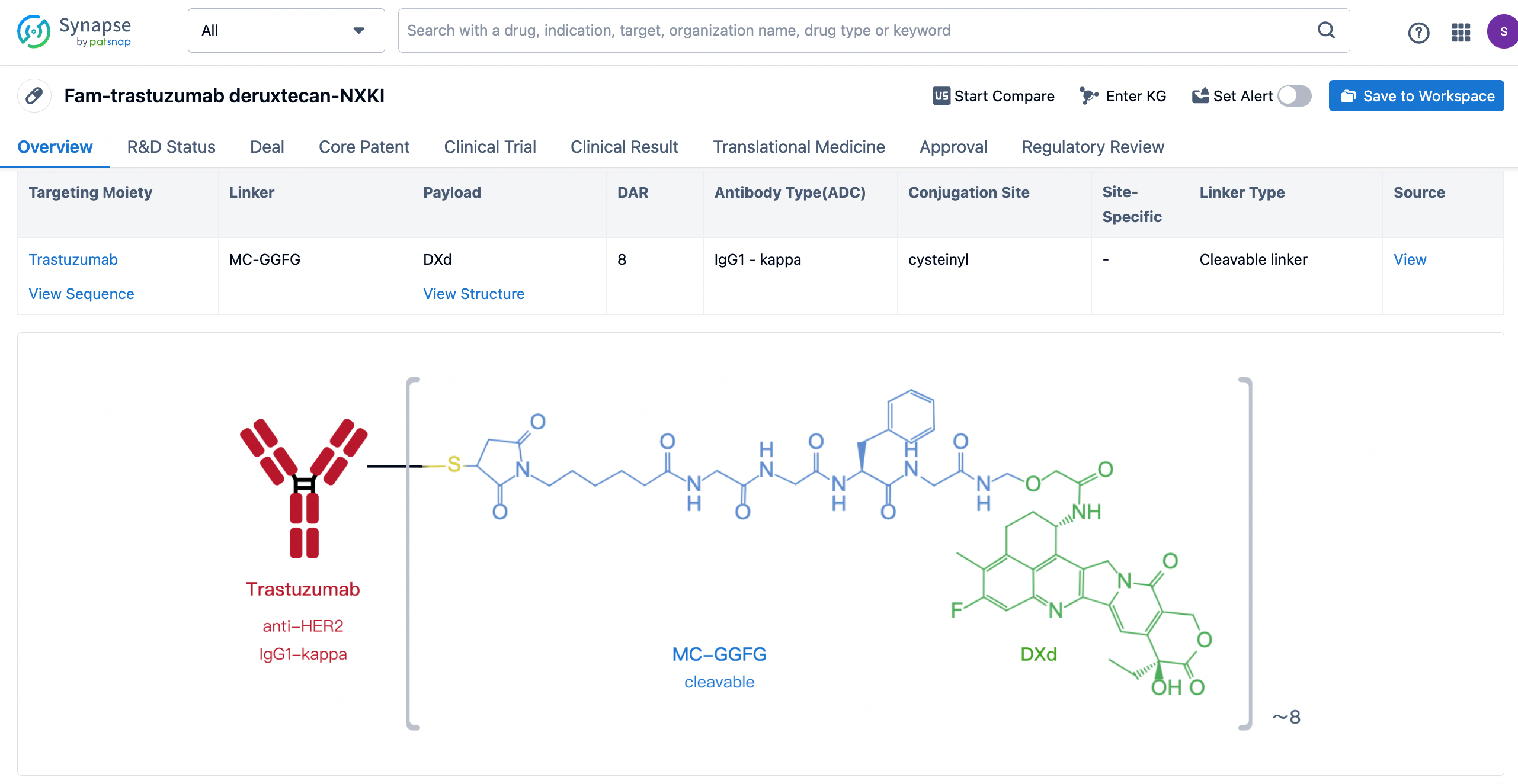
After clicking to enter the drug details page, you can also effortlessly obtain structural information about the ADC drug.
Click on the image below to embark on a brand new journey of drug discovery!