What Does Axicabtagene Ciloleucel Do?
Axicabtagene ciloleucel, branded as Yescarta, is a revolutionary immunotherapy medication developed by Kite Pharma, a subsidiary of Gilead Sciences. It is a CD19-directed, autologous anti-CD19 T cell immunotherapy, which means it uses the patient's own T cells that have been genetically modified to target and attack cancer cells expressing the CD19 protein. This treatment is specifically indicated for adult patients with large B-cell lymphoma who have not responded to or have relapsed after at least two prior treatments. Axicabtagene ciloleucel is part of a new class of cancer therapies known as CAR T-cell therapies and is available under a special program that requires patient registration to understand its risks and benefits.
Mechanism of Action of Axicabtagene Ciloleucel
The mechanism of action of axicabtagene ciloleucel involves a multi-step process that begins with the collection of the patient's T cells through a procedure called leukapheresis. These T cells are then genetically modified in the laboratory to express a chimeric antigen receptor (CAR) that targets CD19, a protein found on the surface of certain B-cell lymphoma cells. The modified T cells are then infused back into the patient, where they multiply and seek out CD19-positive B cells to destroy them. This targeted approach aims to spare healthy cells while providing a potent anti-cancer effect.
How to Use Axicabtagene Ciloleucel
Axicabtagene ciloleucel is administered intravenously as a suspension and is given by trained healthcare professionals in an authorized hospital or clinic. The treatment involves a preparatory chemotherapy regimen known as lymphodepleting chemotherapy, which is administered a few days before the infusion of axicabtagene ciloleucel to help prepare the patient's body for the therapy. Premedication with acetaminophen and diphenhydramine is also given before the infusion to reduce the risk of side effects. The actual infusion of axicabtagene ciloleucel usually takes less than 30 minutes and is given as a one-time treatment. Patients are closely monitored for at least seven days after the infusion for any allergic reactions or serious side effects.
Side Effects of Axicabtagene Ciloleucel
Axicabtagene ciloleucel can cause a range of side effects, including serious and potentially life-threatening conditions. Patients should be alert for signs of an allergic reaction, cytokine release syndrome (CRS), and life-threatening nerve problems. CRS symptoms may include fever, chills, trouble breathing, confusion, severe vomiting or diarrhea, and fast or irregular heartbeats. Nerve problems can manifest as speech issues, memory problems, confusion, or seizures. Common side effects may include nausea, diarrhea, low blood cell counts, confusion, and fast heartbeats. Patients should seek immediate medical attention if any of these symptoms occur.
Drug Interactions with Axicabtagene Ciloleucel
Axicabtagene ciloleucel may interact with other medications, including prescription and over-the-counter drugs, vitamins, and herbal products. It is crucial to inform healthcare providers of all current medications before starting treatment with axicabtagene ciloleucel. Additionally, patients should avoid receiving live vaccines before and after treatment, as the therapy can suppress the immune system. Women of childbearing age should use contraception during treatment and for a specified period after the last infusion, and men should use contraception if their partner is able to become pregnant.
In conclusion, axicabtagene ciloleucel is a groundbreaking therapy for adults with large B-cell lymphoma who have exhausted other treatment options. Its targeted approach using genetically modified T cells offers a new frontier in personalized cancer treatment. However, due to its potent effects and potential for severe side effects, patients receiving axicabtagene ciloleucel must be closely monitored by healthcare professionals throughout the treatment journey.
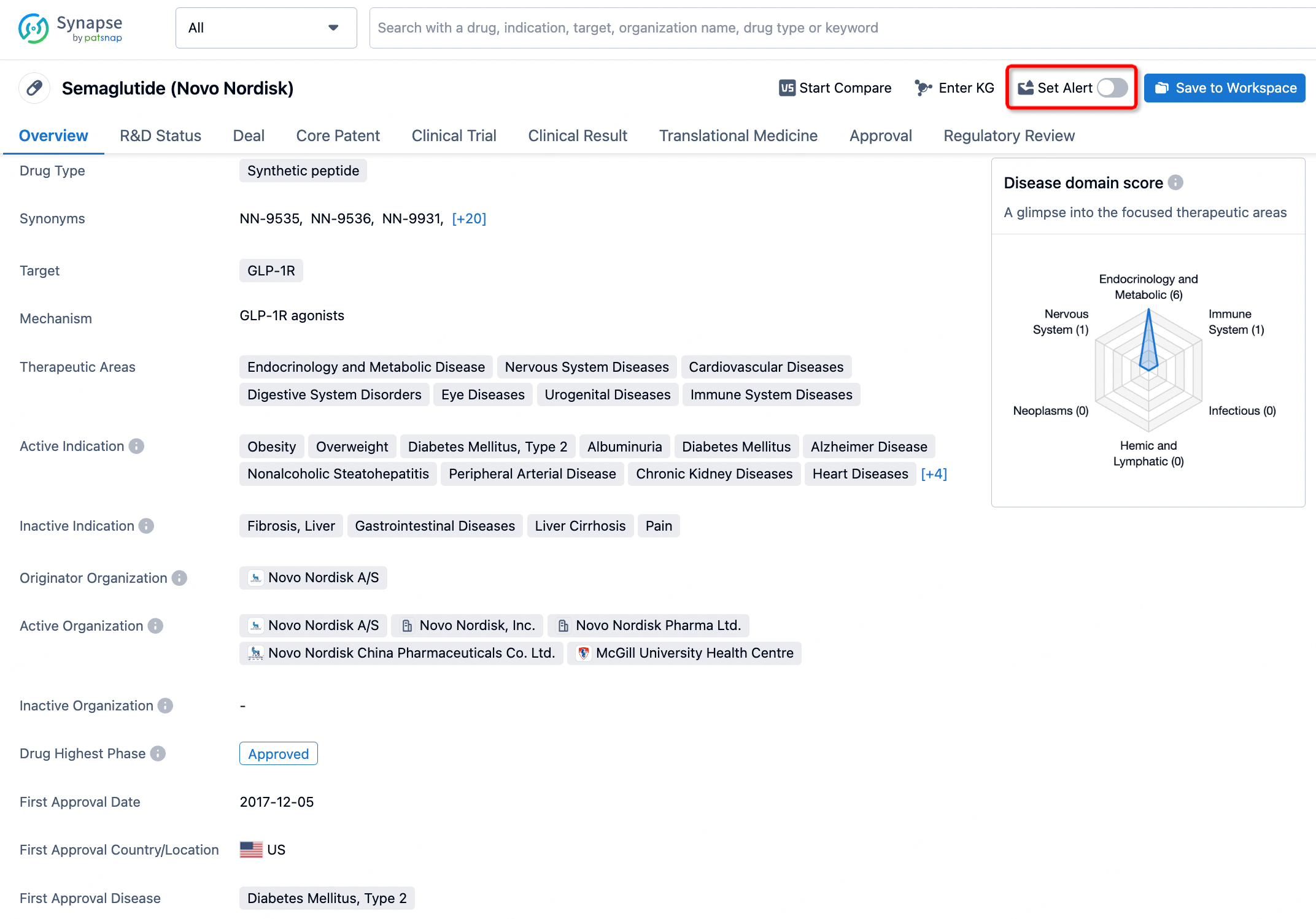
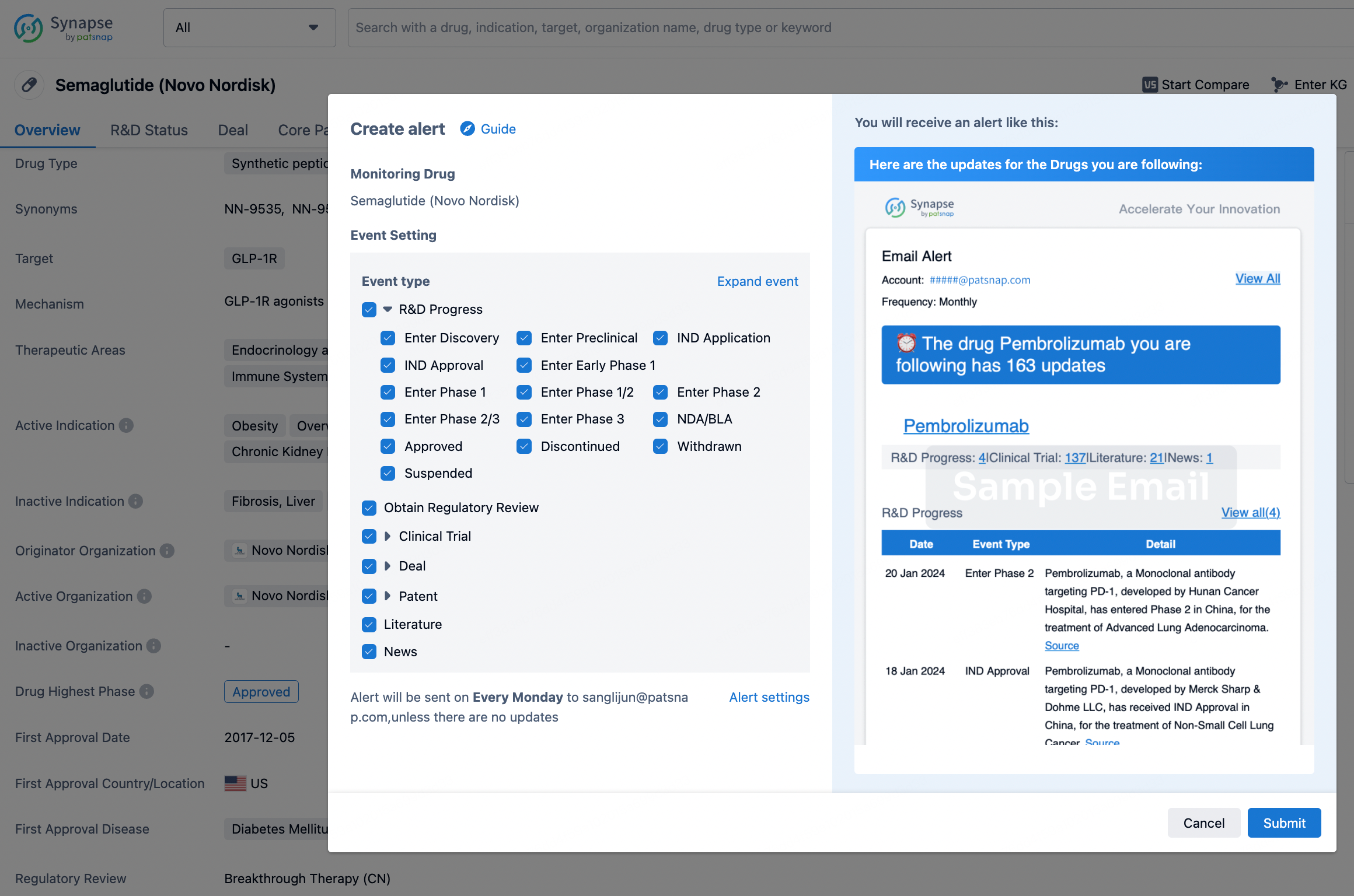
How to obtain the latest development progress of all drugs?
In the Synapse database, you can keep abreast of the latest research and development advances of all drugs anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!