Zealand and Boehringer Ingelheim Collaborate on Latest Development of GLP-1R/GCGR Dual Agonist Survodutide
Recently, Zealand Pharma, a Danish biotechnology company, and Boehringer Ingelheim (BI) announced breakthrough clinical trial results for Survodutide, a novel drug targeting metabolic dysfunction-associated steatohepatitis (MASH). In a secondary analysis of the Phase II trial, 64.5% of F2 and F3 liver fibrosis patients showed improvement in liver fibrosis after receiving Survodutide treatment, without worsening MASH symptoms, compared to 25.9% in the placebo group. Additionally, 52.3% of patients experienced significant improvement in liver fibrosis (from mild to moderate or severe) with Survodutide treatment, compared to 25.8% in the placebo group.
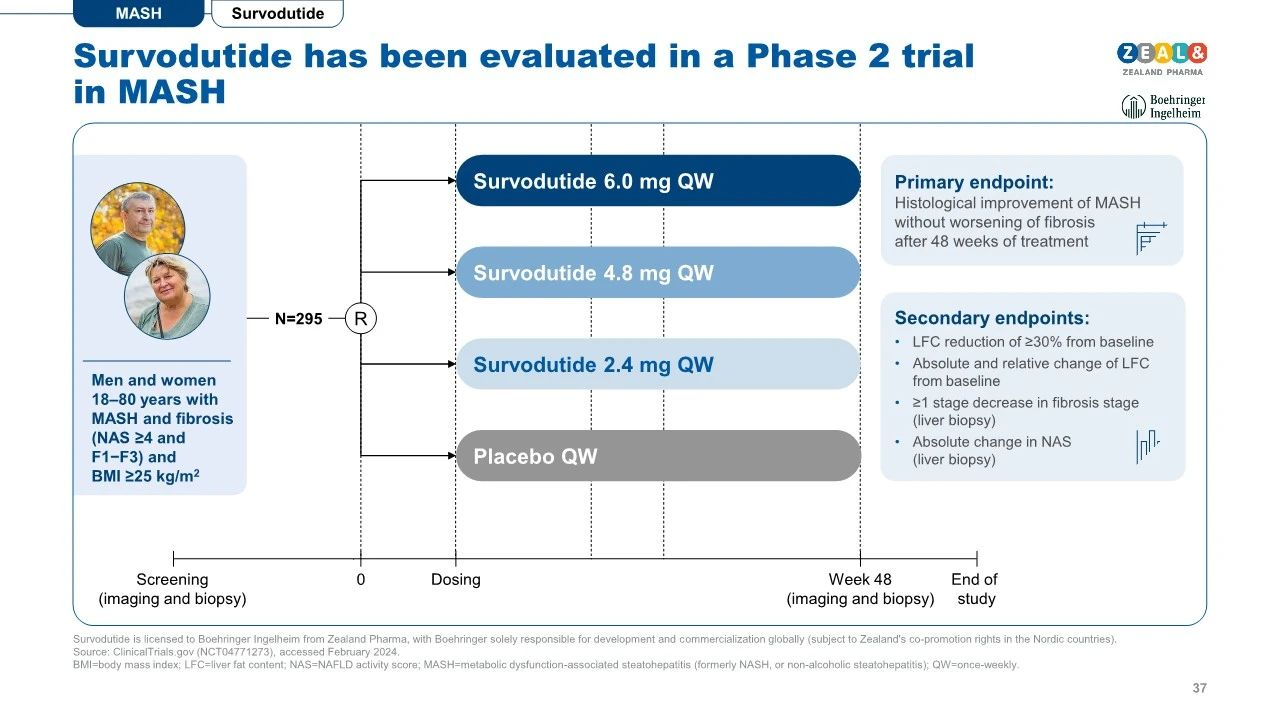
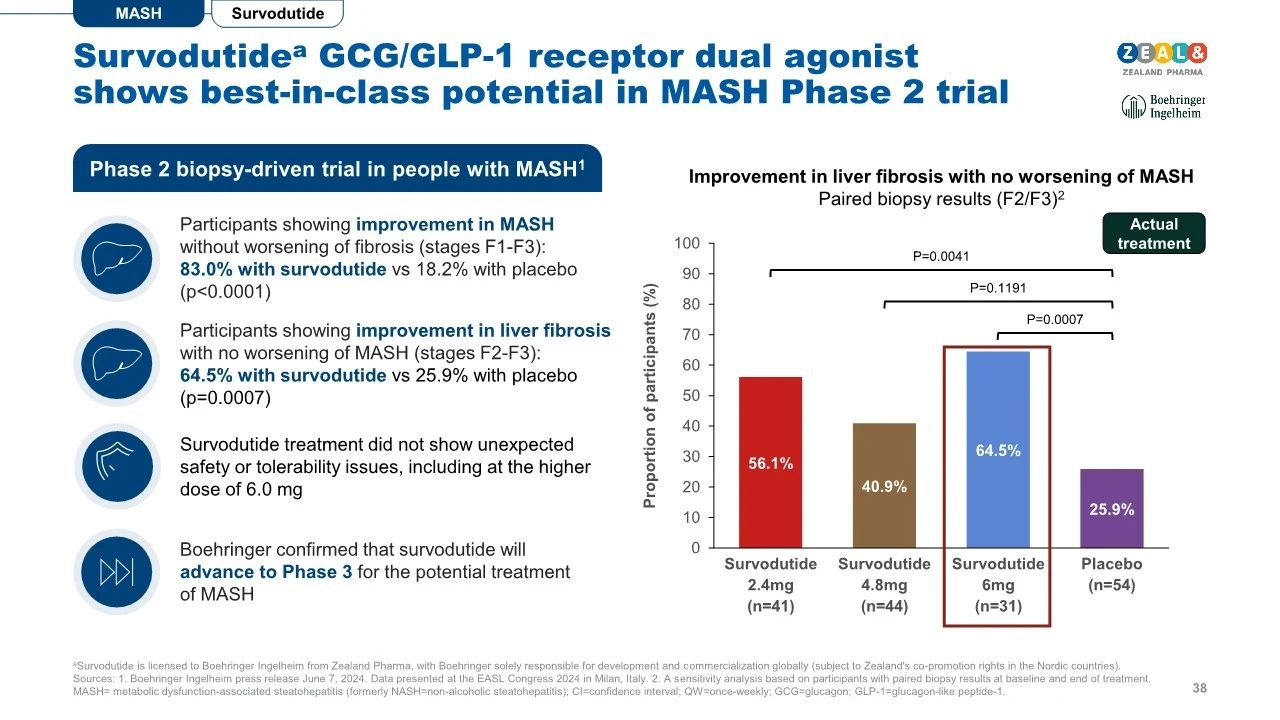
In terms of safety, adverse reactions from Survodutide were similar to those associated with conventional GLP-1R agonists when compared to the placebo, including nausea (66% vs. 23%), diarrhea (49% vs. 23%), and vomiting (41% vs. 4%). The incidence of serious adverse reactions was 8%, compared to 7% with the placebo.
Furthermore, Survodutide will be evaluated in several Phase III studies targeting overweight and obese populations, conditions closely related to MASH. One additional Phase III trial is assessing whether Survodutide can help overweight or obese individuals, as well as diagnosed or suspected MASH patients, reduce liver fat and lose weight. The relevant Phase II clinical data for Survodutide was recently published in the New England Journal of Medicine.
About Survodutide
Survodutide is an acylated long half-life dual agonist for the GCGR/GLP-1 receptor. It works by activating the glucagon and GLP-1 receptors, which are crucial in controlling metabolic functions. In the treatment of MASH and related cardiovascular, renal, and metabolic diseases, Survodutide has shown exceptional potential. It has received fast-track designation from the FDA and was granted priority medicine (PRIME) status by the European Medicines Agency (EMA) in November of last year for the treatment of MASH with fibrosis.
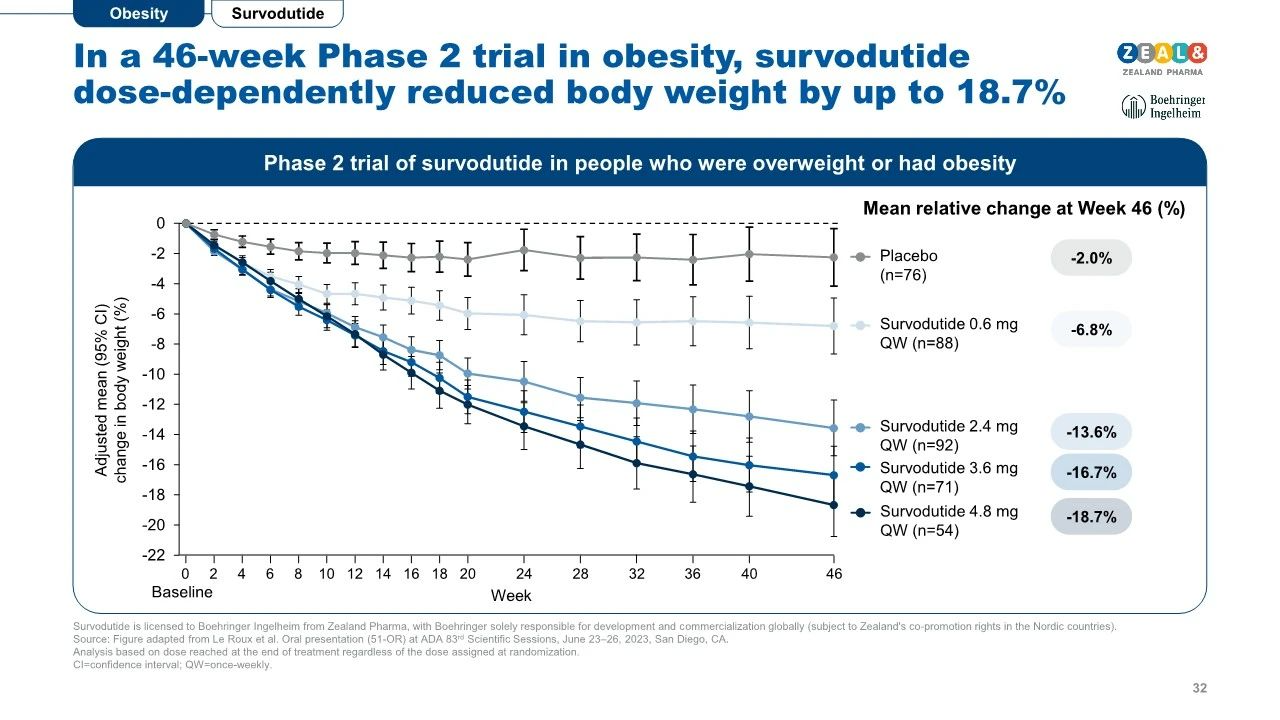
Sixteen-week Phase II clinical data demonstrate that Survodutide continuously reduces glycated hemoglobin and body weight in obese patients.
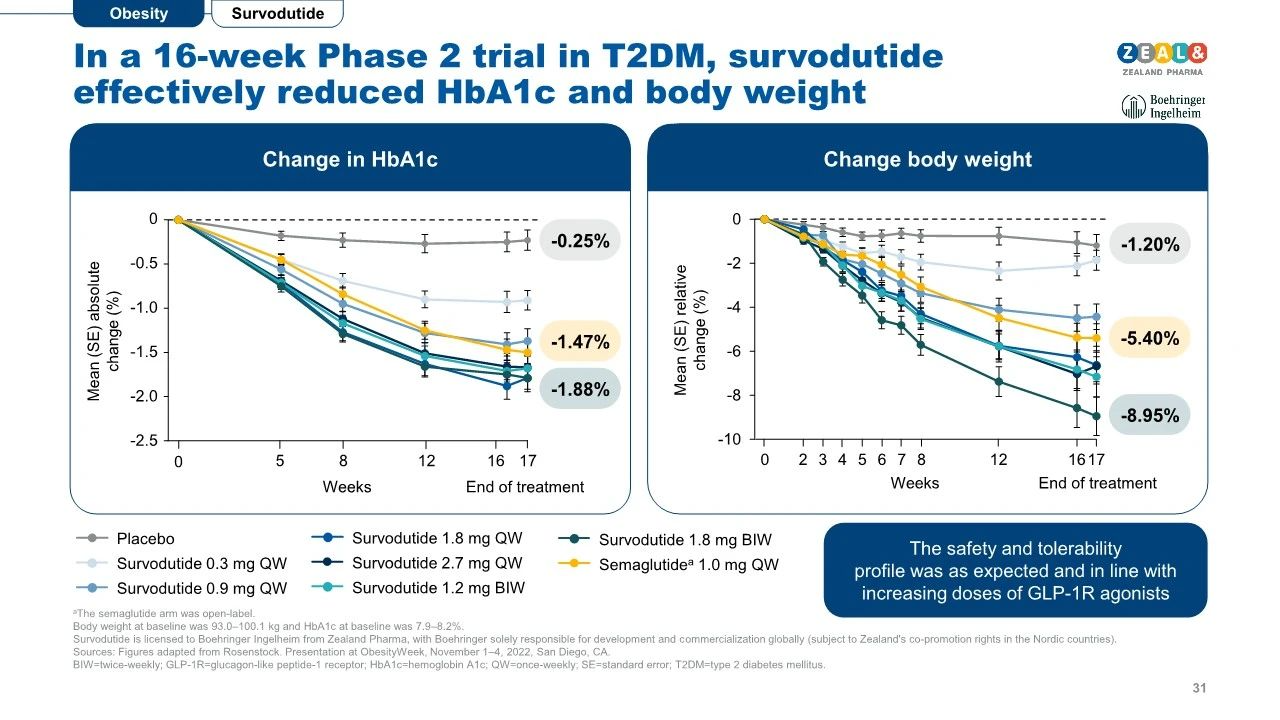 Forty-six-week treatment resulted in up to an 18.7% reduction in body weight, and long-term drug intervention did not reach a maximum body weight reduction.
Forty-six-week treatment resulted in up to an 18.7% reduction in body weight, and long-term drug intervention did not reach a maximum body weight reduction.
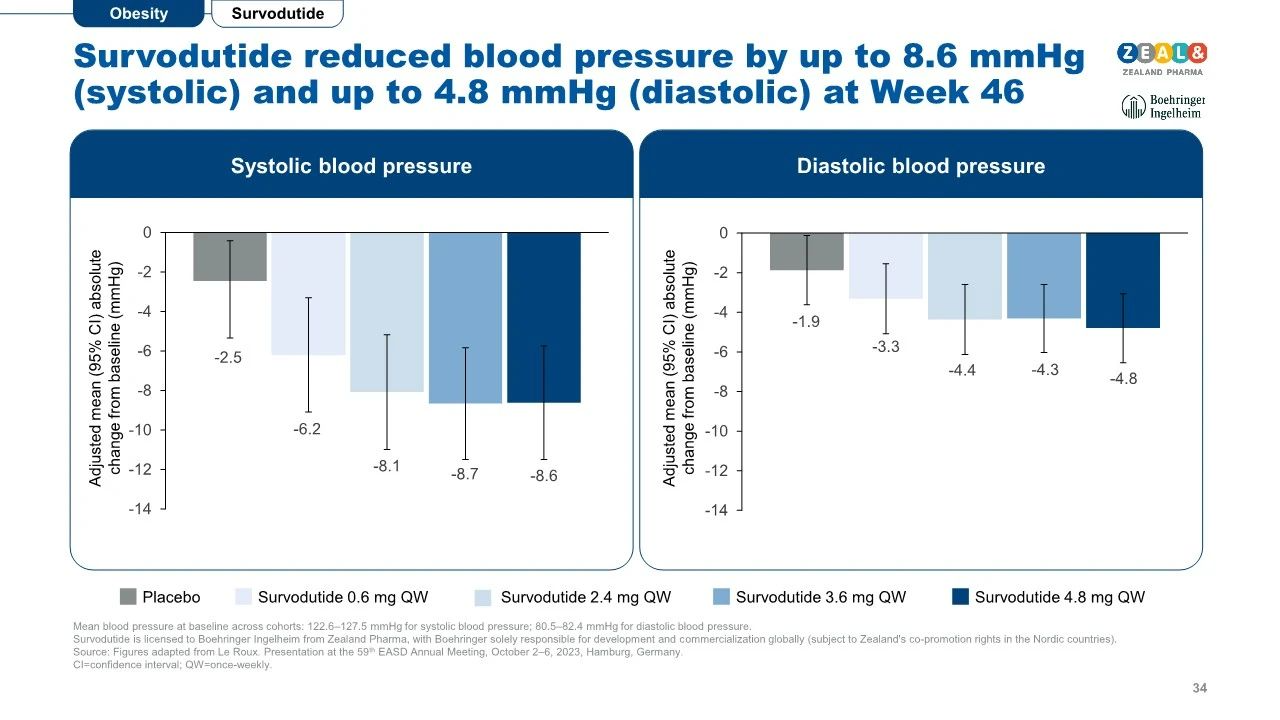
Survodutide treatment also improved blood pressure metrics for patients.
Collaboration between Zealand and BI
The collaboration between Zealand and BI dates back to 2011, when the two parties agreed to develop drug candidates for the treatment of type 2 diabetes and obesity. As part of the agreement, Survodutide was licensed to BI. Currently, Survodutide is under development for the treatment of obesity (Phase III) and MASH (Phase II), with BI exclusively responsible for its global development and commercialization (Zealand retains co-promotion rights in Nordic countries). According to the agreement, Zealand Pharma is eligible to receive up to €315 million in unpaid milestone payments, as well as tiered royalties in the high single digits to low double digits on potential global sales by BI.
About Zealand
Zealand is a Danish biotechnology company with 25 years of expertise in discovering, designing, and developing peptide-based drugs. The company designs peptide analogs to enhance biological activity, extend duration of action, and increase stability, thereby providing innovative and improved treatments for various diseases.
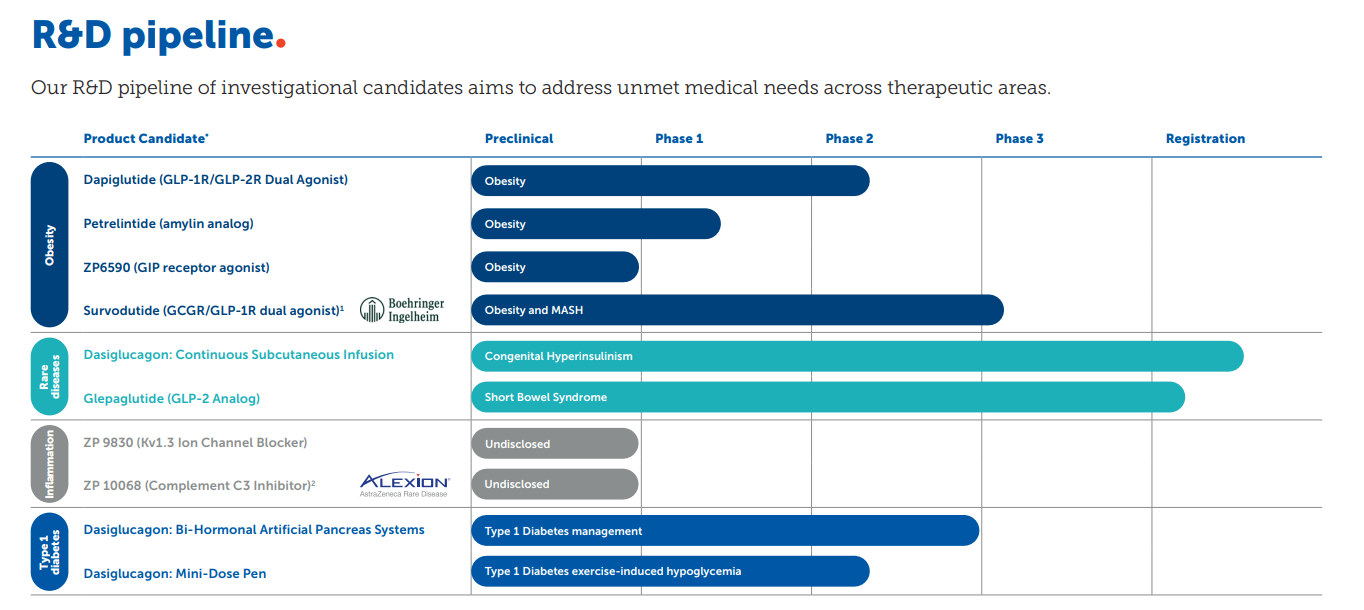
The company's current research pipeline includes:
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!