Zealand Pharma Reports Positive Results from Phase 1b Trial of Long-Acting Amylin Analog Petrelintide
Zealand Pharma A/S, a biotech firm dedicated to inventing and advancing novel peptide-based therapies, has reported favorable top-line data from Part 2 of their Phase 1b trial involving multiple ascending doses. This trial examines the safety, tolerability, and clinical impacts of a 16-week regimen of petrelintide, an extended-release amylin analog being developed for weight control.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 David Kendall, MD, Chief Medical Officer at Zealand Pharma, commented on the findings from this 16-week study, describing them as both exciting and persuasive. The trial demonstrated significant and clinically meaningful reductions in body weight, with excellent tolerability. Kendall expressed confidence in petrelintide's tolerability, suggesting it could be an effective alternative to incretin-based therapies for managing overweight and obesity.
David Kendall, MD, Chief Medical Officer at Zealand Pharma, commented on the findings from this 16-week study, describing them as both exciting and persuasive. The trial demonstrated significant and clinically meaningful reductions in body weight, with excellent tolerability. Kendall expressed confidence in petrelintide's tolerability, suggesting it could be an effective alternative to incretin-based therapies for managing overweight and obesity.
Kendall further mentioned, "These findings lay the foundation for swiftly advancing to Phase 2b trials of petrelintide. They highlight this long-acting amylin analog's potential to achieve weight loss on par with GLP-1 receptor agonists, while offering a more favorable patient experience. We anticipate starting the Phase 2b clinical trial of petrelintide for individuals dealing with overweight and obesity in late 2024."
During the trial, petrelintide showed excellent tolerability, with no serious or severe adverse events reported. Gastrointestinal adverse events were mostly mild, aside from two moderate incidents that led one participant to discontinue after the third dose. Importantly, no other participants discontinued due to adverse events. Vomiting was not reported by any other participants, and there were only two mild cases of diarrhea. Nausea occurred in 16.7-33.3% of the active groups and 16.7% of the placebo group. Injection site reactions were minimal and all mild. No anti-drug antibodies were detected.
These interim results came from a preliminary data analysis, with final outcomes to include complete follow-ups from the third cohort. Zealand plans to present detailed 16-week results from Part 2 of the MAD trial at a scientific conference later this year, while also preparing to advance petrelintide’s clinical development and initiate a Phase 2b trial in late 2024.
Petrelintide (ZP8396) is a long-acting amylin analog intended for once-weekly subcutaneous injection, designed with chemical and physical stability at neutral pH to minimize fibrillation, and enabling co-formulation with other peptides. Produced in the pancreatic beta cells and co-secreted with insulin, amylin responds to ingested nutrients.
Current clinical or preclinical evidence indicates that petrelintide might achieve weight loss comparable to that of GLP-1 receptor agonists, while offering better tolerability and preserving lean mass, thereby enhancing patient experience and delivering high-quality weight loss.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
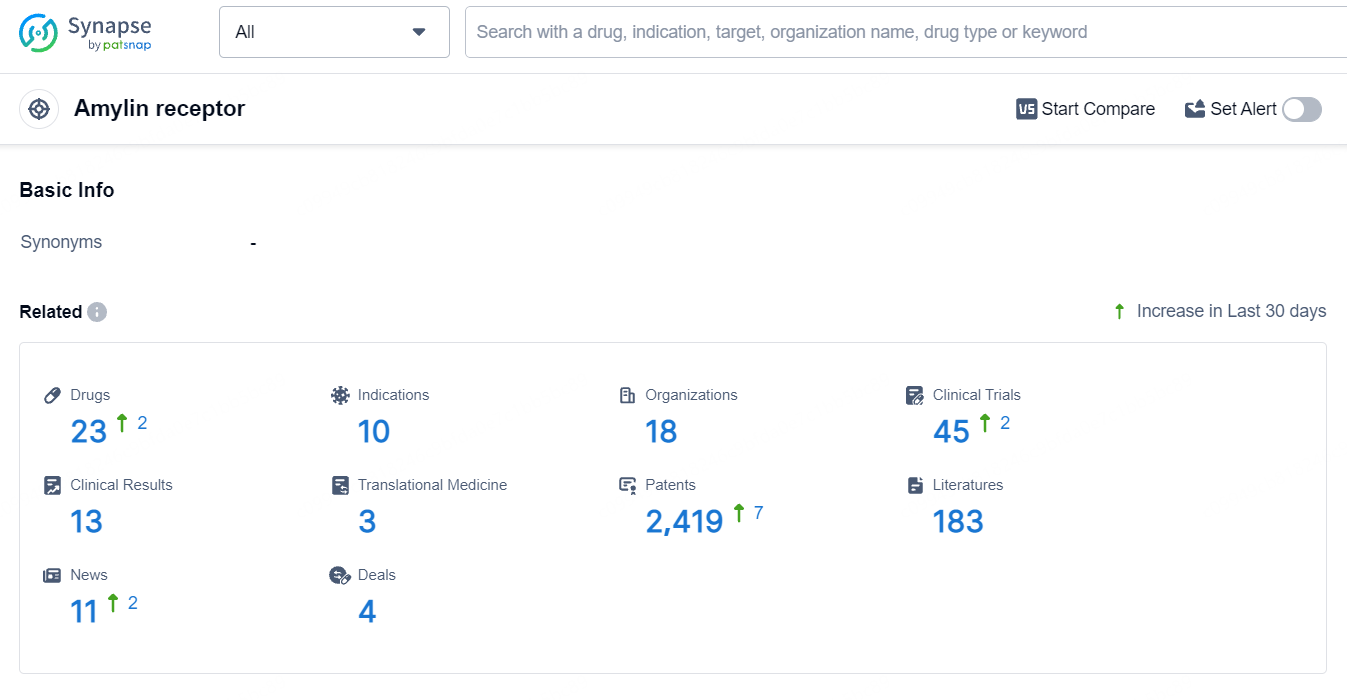
According to the data provided by the Synapse Database, As of June 26, 2024, there are 23 investigational drugs for the amylin receptor target, including 10 indications, 18 R&D institutions involved, with related clinical trials reaching 45, and as many as 2389 patents.
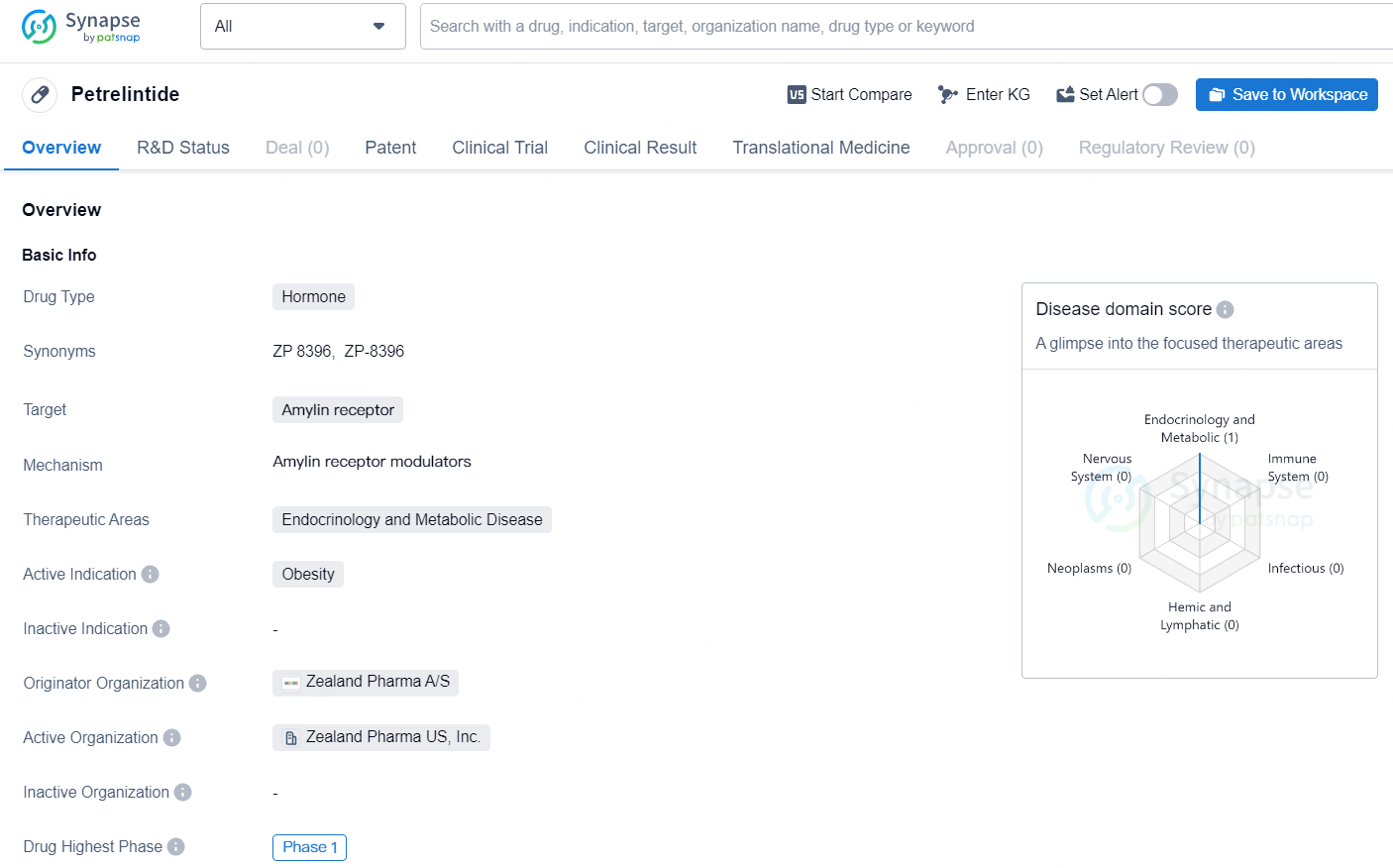
Petrelintide is a hormone drug that targets the amylin receptor and is primarily focused on therapeutic areas within endocrinology and metabolic disease. Petrelintide is a hormone drug targeting the amylin receptor, with a focus on addressing obesity in the therapeutic areas of endocrinology and metabolic disease. Developed by Zealand Pharma A/S, the drug is currently in Phase 1 of development, marking an important stage in its clinical evaluation.