Zenocutuzumab - A Bispecific Antibody Targeting HER2/HER3
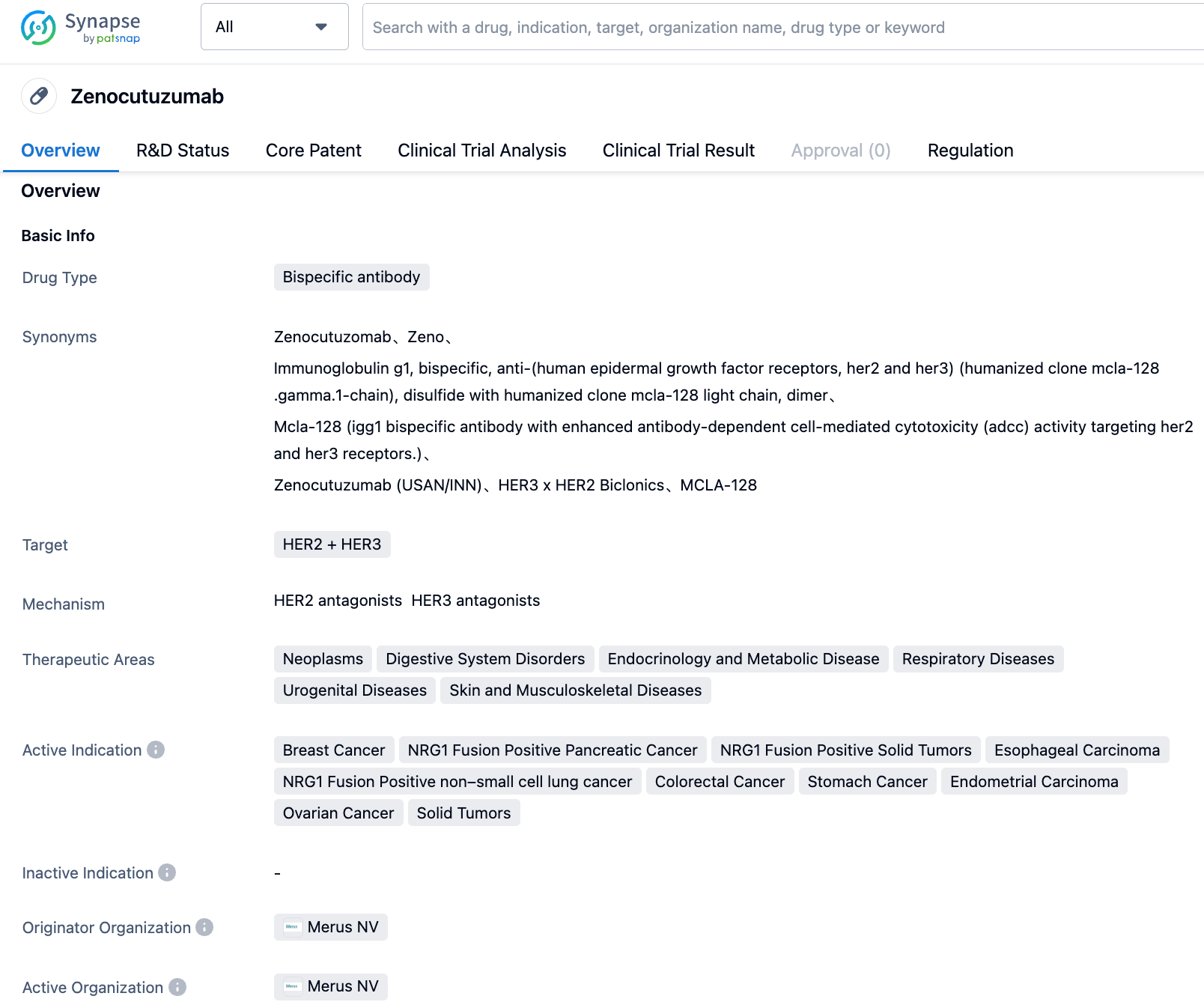
Zenocutuzumab is a bispecific antibody targeting HER2/HER3 developed by Merus NV. On July 7, 2023, the U.S. FDA granted Zenocutuzumab breakthrough therapy desig-nation for NRG1 fusion-positive non-small cell lung cancer.
According to Synapse, this drug is currently adapted for indications including breast cancer, NRG1 fusion-positive pancreatic cancer, NRG1 fusion-positive solid tumors, esophageal cancer, NRG1 fusion-positive non-small cell lung cancer, colorectal cancer, gastric cancer, endometrial cancer, and ovarian cancer. Its highest development stage globally is currently in clinical phase 2, covering most indications such as esophageal cancer, colorectal cancer, and breast cancer.
Although the drug is still in Phase 2 clinical trials, it has received multiple regulations. On July 22, 2020, it received FDA Orphan Drug Designation for pancreatic cancer. On June 30, 2023, for NRG1 fusion-positive pancreatic cancer, it received the FDA Breakthrough Therapy Designation. This is the second Breakthrough Therapy Designation, for NRG1 fusion-positive non-small cell lung cancer. The designation is based on the outstanding clinical trial results of NCT02912949 and NCT04100694.
Mechanism of Action
NRG1 fusion is an emerging pan-cancer target, present in various types of cancer. NRG1 rearrangement is one of the drivers of solid tumor recurrence, with NRG1 binding to HER3, promoting heterodimerization with other HER/ERBB kinases, enhancing downstream signal transduction and tumor occurrence. Zenocutuzumab is an antibody-dependent, enhanced cytotoxic HER2/HER3 bispecific antibody, which can inhibit the phosphorylation of HER3 and AKT, induce the expression of apoptosis markers and inhibit cell growth.
Core Clinical Trials
NRG1 Fusion Positive Solid Tumors: In the Phase 2 clinical trial NCT02912949, the primary endpoint was the INV-assessed objective response rate (ORR) and the secondary endpoint was the duration of response (DOR). The results showed that for participants treated with zenocutuzumab, the ORR was 34, and the median DOR was 9.1 months. This indicates that regardless of the histological type of tumor, zenocutuzumab demonstrated powerful and lasting efficacy for patients with NRG1 fusion-positive cancer, and zenocutuzumab has good tolerability.
Competitive Landscape
According to statistics, there are currently 13 pipeline drugs involving HER2 + HER3, among which the fastest developing one is Tesevatinib (Phase 3), a multi-target drug developed by Exelixis, Inc. Zenocutuzumab's main competitors is Sapitinib developed by AstraZeneca, with targets of EGFR + HER2 + HER3. It is in Phase 2.