Lotilaner - The First Drug for the Treatment of Demodex Blepharitis
Lotilaner (Xdemvy), a small molecule drug that targets GluCls, was developed by Tarsus Pharma-ceuticals, Inc. and received FDA approval on July 24th, 2023 for the treatment of demodex blepharitis.
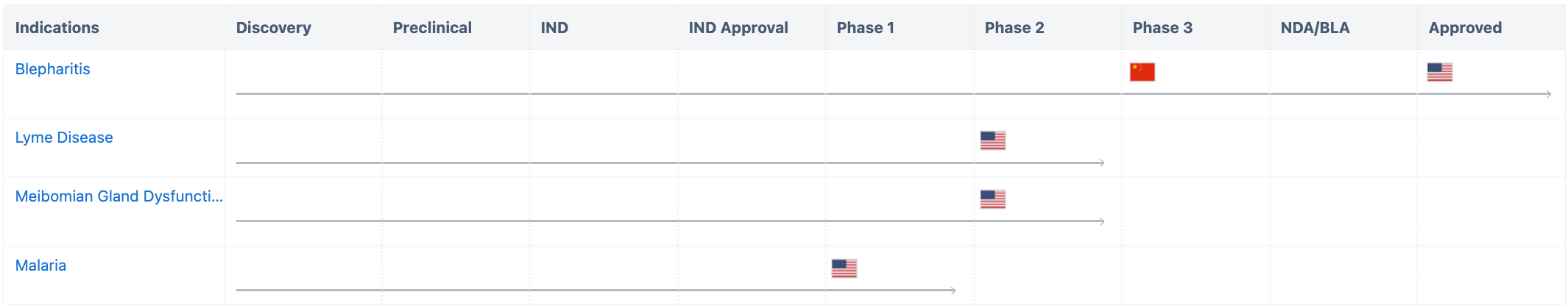
According to Synapse, in addition to blepharitis, Lotilaner is also being researched for its applications in meibomian gland dysfunction, Lyme disease, and malaria. The clinical phase 3 trial for blepharitis has already been conducted in China, while other indications are primarily being developed in the United States.
Xdemvy , formerly known as TP-03, is the first and only FDA approved treatment to directly target Demodex mites, the root cause of Demodex blepharitis.
Blepharitis is a common lid margin disease that is characterized by eyelid margin inflammation, redness and ocular irritation. Demodex blepharitis is caused by an infestation of Demodex mites, the most common ectoparasite found on humans and accounts for over two-thirds of all blepharitis cases.
Mechanism of Action
GluCls (Glutamate-gated chloride ion channels) are ion channel receptors in the nervous system. In various invertebrates, including mites, these ion channel receptors are involved in the transmission of nerve signals. When activated, the receptors open, allowing chloride ions to flow across the cell membrane, resulting in membrane hyperpolarization and further blocking the propagation of nerve impulses. Lotilaner, as a targeted drug for GluCls, can paralyze demodex mites, ultimately causing their death.

Core Clinical Trials
The FDA approval is based on results from two randomized, multicenter, double-masked, vehicle-controlled studies (Saturn-1 and Saturn-2), designed to evaluate the safety and efficacy of Xdemvy in 833 patients, 415 of which received Xdemvy . Patients with Demodex blepharitis were randomized to either Xdemvy or vehicle at a 1:1 ratio and dosed twice daily in each eye over the course of 6 weeks.
Efficacy was demonstrated by a significant improvement in eyelids (reduction of collarettes, the pathognomonic sign of the disease, to no more than 2 collarettes per upper lid) in each study by Day 43, with some patients seeing improvement as early as 2 weeks. Additionally, the endpoints of mite eradication (mite density of 0 mites per lash) and erythema cure (Grade 0) showed statistically significant improvement at Day 43 across both studies. In clinical trials, Xdemvy was generally safe and well tolerated. The most common ocular adverse reactions observed in the studies were instillation site stinging and burning which was reported in 10% of patients. Other ocular adverse reactions reported in less than 2% of patients were chalazion/hordeolum (stye) and punctate keratitis.
Competitive Landscape
According to statistics, there are currently only three pipeline drugs targeting GluCls, all of which have been approved for marketing. In addition to Lotilaner, there is Moxidectin developed by Pfizer Inc., and Ivermectin developed by Merck Sharp & Dohme Corp. The molecular structure diagrams of the three drugs are shown below.