Progress in the Research and Development of PI3Kα Drug Targets
PI3Kα is a crucial lipid kinase and one of the most frequently mutated kinases in cancer. It consists of a heterodimer composed of the catalytic subunit p110α and the regulatory subunit p85α. It is closely associated with cell growth and proliferation, and its acquired functional mutations serve as driving factors in tumor formation, making it a significant target for cancer treatment.
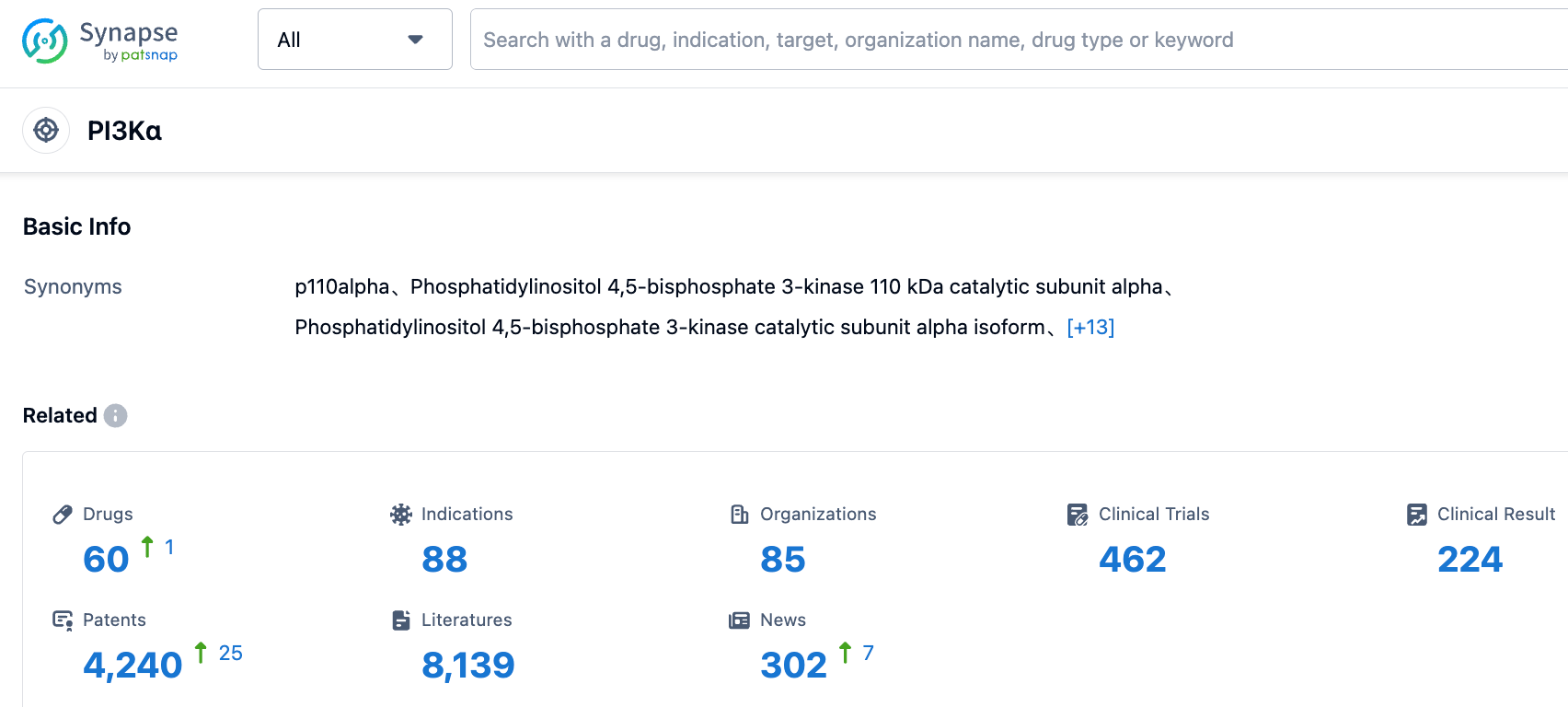
In the field of PI3Kα targeted drug development, data from the Synapse database shows that there are currently 60 drugs being developed that target PI3Kα. These drugs cover 88 indications, with 85 development organizations actively participating in research and competition in this field, highlighting its importance in pharmaceutical research. Additionally, 462 clinical trial results, 4240 patents, and 8139 literature have further strengthened the feasibility and research progress of PI3Kα-targeted drugs. These data reflect the level of activity in the field of PI3Kα-targeted drugs and its future development potential.
Competition Landscape of PI3Kα
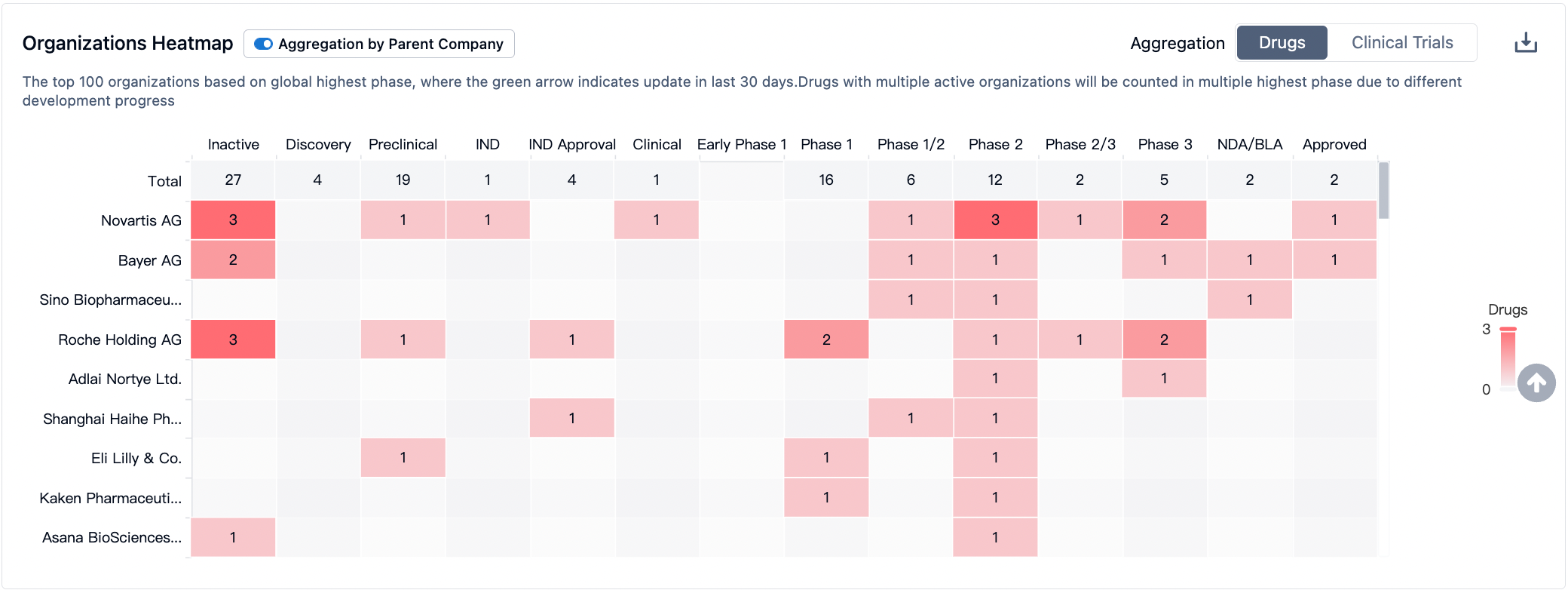
In the R&D of PI3Kα targeted drugs, large pharmaceutical companies such as Novartis, Bayer, and Roche are actively participating. Novartis and Bayer have been successful in launching Alpelisib and Copanlisib respectively, demonstrating high market competitiveness. The active participation of these companies has intensified the competition in PI3Kα targeted drugs sector, offering hopes for more treatment options for patients.
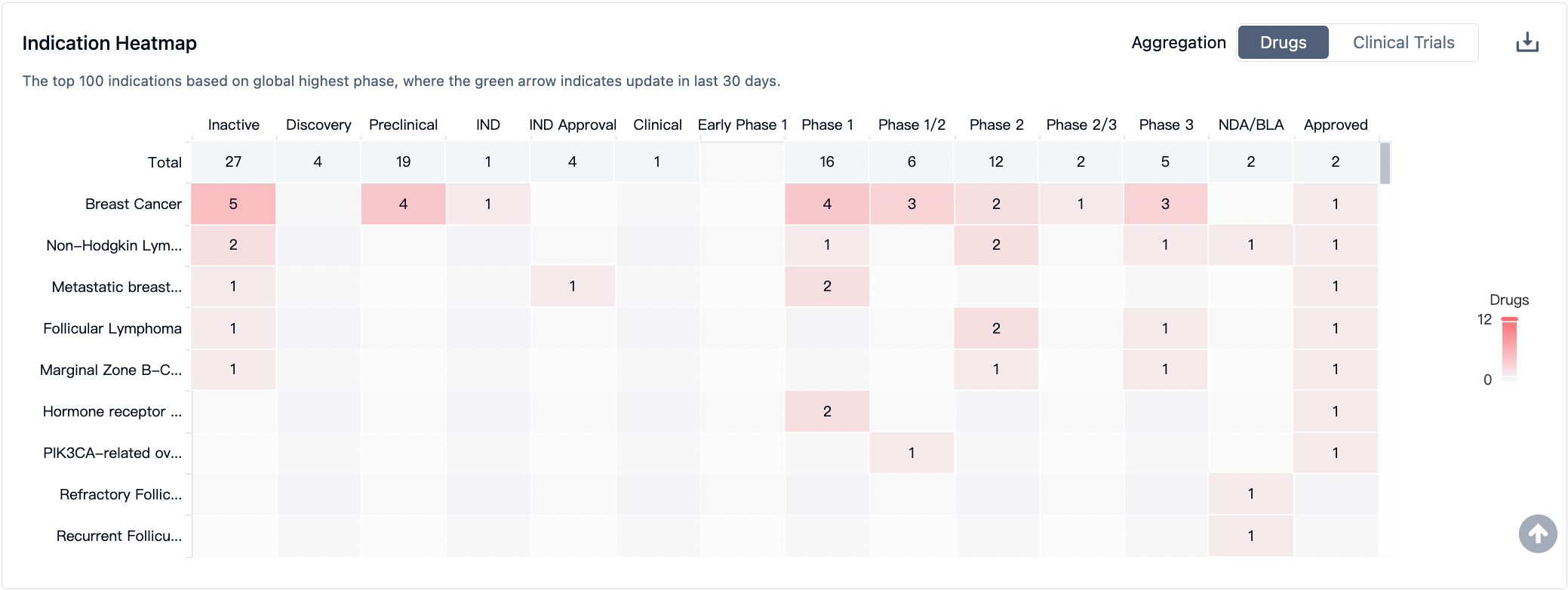
The development of PI3Kα-targeted drugs for various indications has made positive progress, generating widespread attention. In the field of breast cancer, 19 drugs are currently under development, demonstrating the potential and research investment in this area. These data reflect the broad application prospects of PI3Kα-targeted drugs in multiple tumor types, while also highlighting the enthusiasm and efforts of different research institutions in developing drugs for specific indications. With further research and the conduct of clinical trials, it is expected that more PI3Kα-targeted drugs will be introduced, bringing new breakthroughs and hopes for the treatment of these diseases.
Key Drug
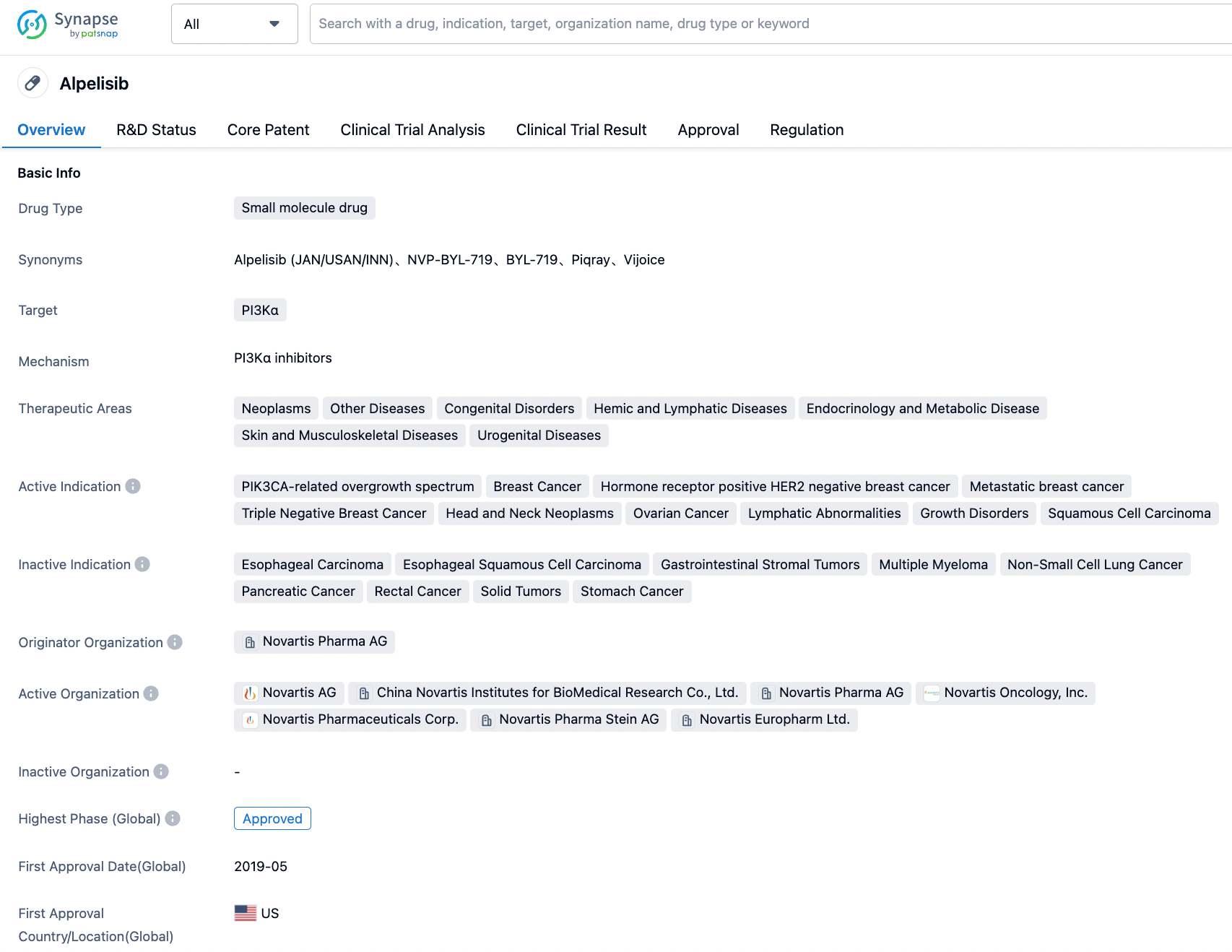
Alpelisib, developed by Novartis, is an orally available small molecule alpha-specific PI3K inhibitor. On May 24, 2019, the U.S. FDA approved the combined use of alpelisib and the endocrine therapy fulvestrant for the treatment of patients with HR+/HER2- advanced or metastatic breast cancer carrying PIK3CA gene mutations. This is the first PI3K inhibitor approved by the FDA for the treatment of breast cancer, and also the first New Molecular Entity (NME) approved under the FDA's Real-Time Oncology Review (RTOR) pilot program. This approval validates the safety and efficacy of Piqray in clinical practice.
RLY-2608 is known as the first pan-allosteric variant (H1047X, E542X, and E545X), and as an allosteric selective PI3Kα inhibitor designed to overcome these limitations. RLY-2608 is currently under clinical trial, aiming to treat patients with advanced solid tumors carrying PIK3CA (PI3Kα) mutations.
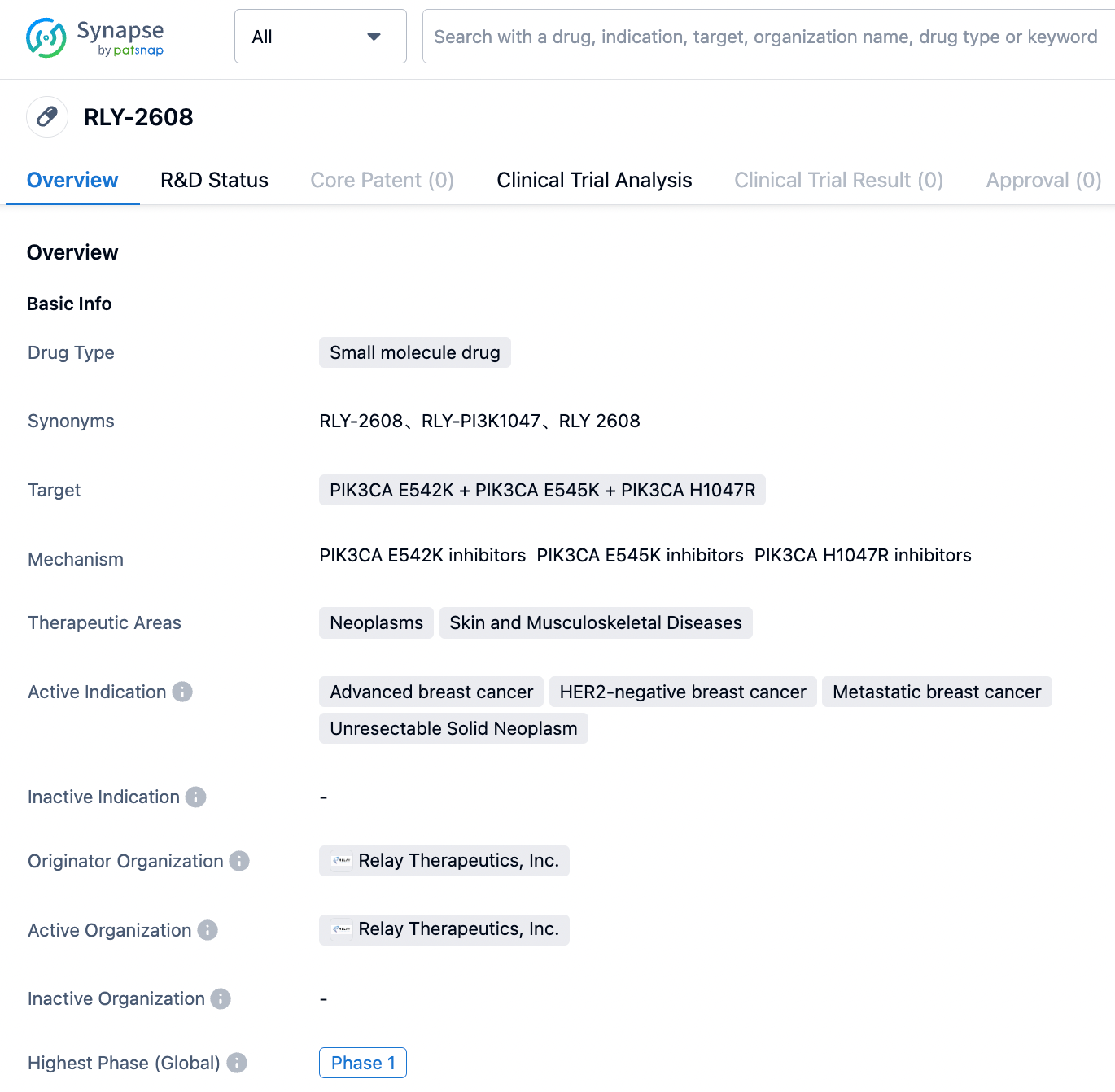
Based on the preclinical data of RLY-2608, as can be seen in the following Figures A and B, the KD calculations evaluated by Surface Plasmon Resonance (SPR) demonstrate that RLY-2608 preferentially binds to mutated proteins, compared to Alpelisib. Moreover, buparlisib, an orthosteric site inhibitor, cannot eliminate this binding, proving that RLY-2608 has generated a new binding site. Figure C indicates that RLY-2608 binds to the mutated proteins more quickly. Figure D evidences that RLY-2608 has superior biochemical selectivity for mutant PI3Kα than WT and other family subtypes. The kinase spectrum in Figure E shows that RLY-2608 has extremely high kinase selectivity, reducing the possibility of off-target side effects.
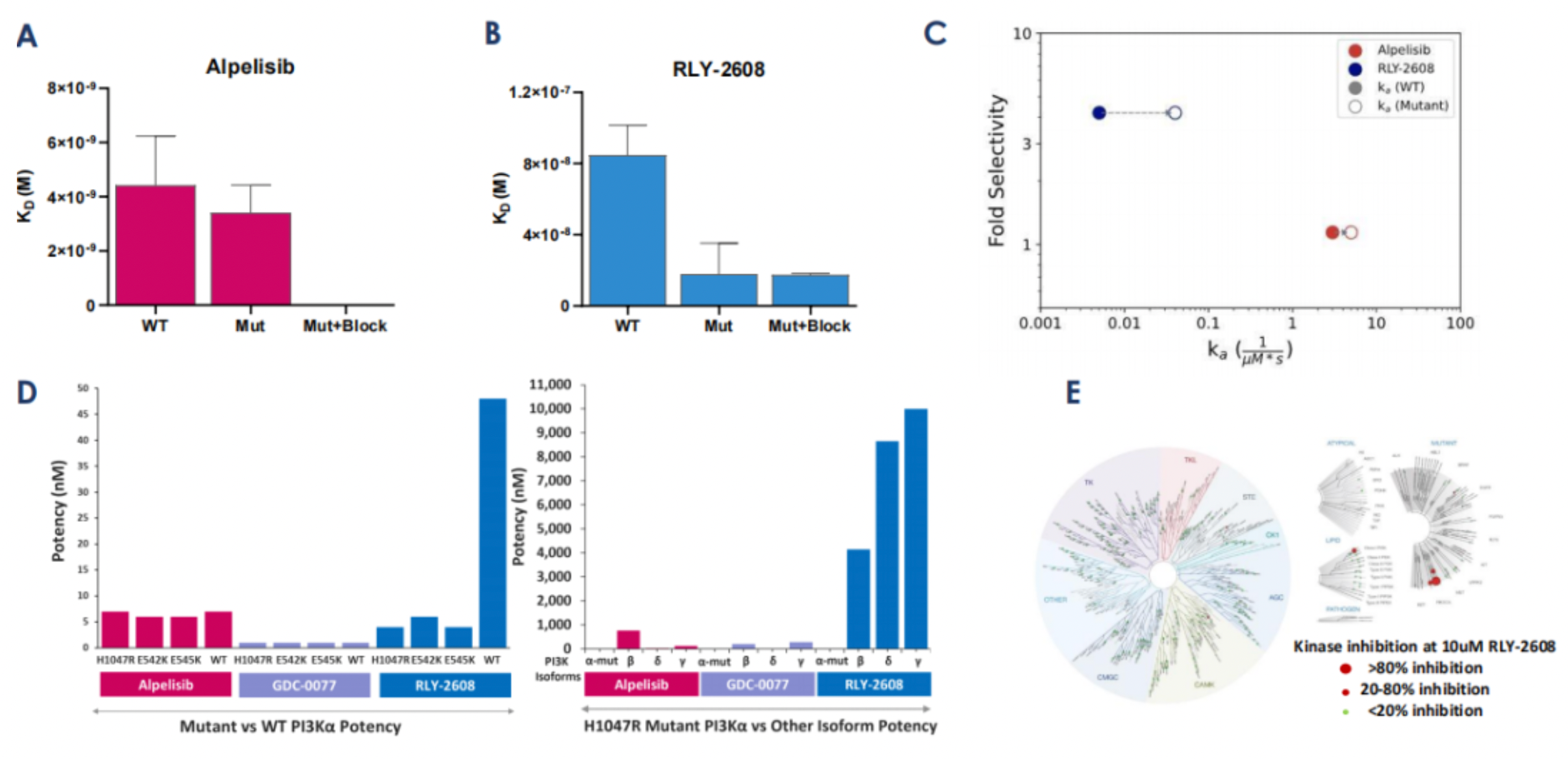
As shown in Figure A and B, RLY-2608 inhibits phosphorylated AKT (pAKT) in PIK3CA mutant cancer cell lines, regardless of hotspot mutations. After extended incubation (5 days), RLY-2608 can inhibit proliferation. Figure D shows that RLY-2608 displays effective tumor growth inhibition in a dose-dependent manner, and can achieve complete regression at doses with smaller impact on insulin levels, compared to positive control inhibitors. However, pharmacological data in Figure C indicates that, at equivalent doses, RLY-2608 does not demonstrate better tumor growth inhibition (compared to GDC-077 and Alpelisib). This could be because other PI3K isoforms such as δ can act as anti-tumor agents via immune regulation. As a single PI3Kα inhibitor, RLY-2608 theoretically sacrifices potency to enhance safety.
In other disclosed data, RLY-2608 achieved maximum therapeutic effects in both kinase and helical structural domain PIK3CA mutated heterologous transplantation models, significantly reducing the rise in insulin levels compared to conformational inhibitors. Across different species, the exposure of RLY-2608 dosage exceeded the mutated PI3Kα cell PD IC90, without causing an increase in glucose levels or histopathological changes associated with glucose metabolism disorders.
These pre-clinical results support the clinical study of RLY-2608 as a mutated PI3Kα inhibitor.
Currently, RLY-2608 is under investigation in a Phase 1 clinical trial. This is an open-label First-in-Human (FIH) study aimed at evaluating the Maximum Tolerated Dose (MTD), safety, tolerability, Pharmacokinetics (PK), pharmacodynamics, and preliminary anti-tumor activity of RLY-2608 in patients with late-stage solid tumors harboring PIK3CA mutations in blood and/or tumor.
There is an increasing breakthrough in clinical trials of PI3Kα inhibitors, but the high toxicity and other side effects brought by PI3Kα inhibitors also pose a significant challenge for clinical application. Traditionally, the development of PI3Kα inhibitors has primarily focused on the active site or allosteric site. Therapeutic indications of allosteric inhibitors are limited by the lack of clinically meaningful mutant and wild type (WT) PI3Kα selectivity and pan-isomeric activity. More progress in drug clinical trials is expected to bring blessings to patients.