Request Demo
Last update 18 Oct 2025
Patritumab
Last update 18 Oct 2025
Overview
Basic Info
Drug Type Monoclonal antibody |
Synonyms AMG-888, U3-1287 |
Target |
Action antagonists |
Mechanism HER3 antagonists(Receptor tyrosine-protein kinase erbB-3 antagonists) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization- |
Drug Highest PhaseDiscontinuedPhase 3 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Sequence Code 193897L

Source: *****
Sequence Code 9984986H

Source: *****
Related
11
Clinical Trials associated with PatritumabNCT02633800
Randomized, Placebo-controlled, Double-blind Phase 2 Study of Patritumab (U3-1287) in Combination With Cetuximab Plus Platinum-based Therapy in First Line Setting in Subjects With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck
This study will test an investigational study drug called patritumab. It is a 'randomized study' which means participants have an equal chance of being assigned to receive the experimental medication (patritumab) or a substance that looks like the experimental product, but is not (placebo). Patritumab may work when combined with other medications that are approved for the treatment of head and neck cancer. They are called cetuximab, cisplatin or carboplatin. All participants will receive the other medications approved for treatment of head and neck cancer, even if they do not receive the experimental product.
Start Date22 Dec 2015 |
Sponsor / Collaborator |
NCT02350712
An Open Label, Phase 1B Safety Evaluation of Patritumab (U31287) in Combination With Cetuximab Plus Platinum Containing Therapy In Subjects With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck
The purpose of this study is to test a study drug called patritumab. Patritumab may work when combined with other medications that are approved in the UK for treating Squamous Cell Carcinoma of the Head and Neck (SCCHN), called cetuximab, cisplatin or carboplatin. It is hoped that patritumab may have some benefit in treating patients with cancer. This study will help identify how much patritumab can be given in combination with cetuximab, and cisplatin or carboplatin. This study will show how safe and how well tolerated patritumab is when these medications are given together.
Start Date01 Dec 2014 |
Sponsor / Collaborator |
NCT02134015
Phase 3, Randomized, Placebo-Controlled, Double-Blind, Multi-Center, Two-Part Study of Patritumab (U3-1287) In Combination With Erlotinib in EGFR Wild-type Subjects With Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) Who Have Progressed on at Least One Prior Systemic Therapy
1. Part A: Subjects will receive Patritumab or placebo with erlotinib. Progression-free survival will be the primary outcome. Subjects will need to have Epidermal Growth Factor Receptor (EGFR) wild-type, locally advance or metastatic NSCLC and have their cancer progressed after at least one prior systemic anti-cancer therapy, available recent or archival tumor specimen and may not have had previous EGFR-targeted regimen, anti-HER2 (Human Epidermal Growth Factor Receptor 2), anti-HER3, or anti-HER4 therapy. Subjects may have high heregulin or low heregulin.
2. Part B: Subjects will receive Patritumab or placebo with erlotinib. Overall survival will be the primary outcome. Subjects will need to have EGFR wild-type, locally advance or metastatic NSCLC and have their cancer progressed after at least one prior systemic anti-cancer therapy, available recent or archival tumor specimen and may not have had previous EGFR-targeted regimen, anti-HER2, anti-HER3, or anti-HER4 therapy. Only subjects with high heregulin will be enrolled.
2. Part B: Subjects will receive Patritumab or placebo with erlotinib. Overall survival will be the primary outcome. Subjects will need to have EGFR wild-type, locally advance or metastatic NSCLC and have their cancer progressed after at least one prior systemic anti-cancer therapy, available recent or archival tumor specimen and may not have had previous EGFR-targeted regimen, anti-HER2, anti-HER3, or anti-HER4 therapy. Only subjects with high heregulin will be enrolled.
Start Date01 Mar 2014 |
Sponsor / Collaborator |
100 Clinical Results associated with Patritumab
Login to view more data
100 Translational Medicine associated with Patritumab
Login to view more data
100 Patents (Medical) associated with Patritumab
Login to view more data
38
Literatures (Medical) associated with Patritumab01 Sep 2025·JOURNAL OF CLINICAL ONCOLOGY
Article
Author: Murakami, Haruyasu ; Sternberg, David W. ; Fan, Pang-Dian ; Kim, Dong-Wan ; Johnson, Melissa L. ; Massarelli, Erminia ; Felip, Enriqueta ; Steuer, Conor E. ; Fujimura, Masahiro ; Gold, Kathryn A. ; Truchon, Karine ; Nishio, Makoto ; Hayashi, Hidetoshi ; Baik, Christina S. ; Smit, Egbert F. ; Su, Wu-Chou ; Su, Xin ; Jänne, Pasi A. ; Kim, Sang-We
PURPOSE:
Patritumab deruxtecan (HER3-DXd; MK-1022) is an investigational HER3-directed antibody-drug conjugate composed of a human immunoglobulin G1 monoclonal antibody to HER3 (patritumab) covalently linked via a stable tetrapeptide-based cleavable linker to a topoisomerase I inhibitor payload that has shown durable antitumor activity in previously treated patients with
EGFR
-mutated advanced non–small cell lung cancer (NSCLC). We extend these observations to patients with advanced NSCLC with other/no identified driver genomic alterations.
METHODS:
Patients with advanced squamous or nonsquamous NSCLC without a common
EGFR
-activating mutation whose disease had progressed on previous therapies including platinum-based chemotherapy, immune checkpoint inhibitors, and targeted therapy (for patients with known actionable genomic alterations) received HER3-DXd 5.6 mg/kg intravenously once every 3 weeks. The primary end point was confirmed objective response rate (cORR).
RESULTS:
Forty-seven patients were treated with HER3-DXd (median treatment duration, 4.2 [range, 0.7-19.8] months). The cORR was 27.7% (95% CI, 15.6% to 42.6%), and the median duration of response was 8.1 (95% CI, 4.2 to not evaluable) months. The median progression-free survival was 5.5 (95% CI, 4.0 to 11.2) months, and the median overall survival was 15.2 (95% CI, 10.8 to 17.7) months. Similar efficacy was observed in patients with NSCLC harboring identified driver genomic alterations and in those without such genomic features. The rate of study drug discontinuation associated with treatment-emergent adverse events (TEAEs) was 12.8%. Study drug–related grade ≥3 TEAEs occurred in 51.1% of patients and were serious in 12.8% (none were associated with death). Adjudicated treatment-related interstitial lung disease occurred in five patients (10.6%; all grade 1 or 2).
CONCLUSION:
The previously reported efficacy and safety of HER3-DXd in heavily pretreated patients with
EGFR
-mutated NSCLC are also observed in those with other NSCLC subtypes and warrant further clinical evaluation.
01 May 2025·REVUE DES MALADIES RESPIRATOIRES
Pneumopathies interstitielles diffuses induites par les anticorps conjugués
Review
Author: Sagan, C ; Maurier, L ; Pons-Tostivint, E ; Chéné, A-L ; Hulo, P ; Chen, J
INTRODUCTION:
Antibody-drug conjugates (ADCs) represent a promising new therapeutic class in non-small-cell lung cancer (NSCLC) patients. Studies assessing ADC have highlighted a pulmonary toxicity profile in the form of interstitial lung disease (ILD).
STATE OF THE ART:
Several ADCs for NSCLC are currently being developed. In studies evaluating Trastuzumab-Deruxtecan (Her-2 target), incidence of drug-induced ILD ranged from 10.7 to 26.0%, and from 3.6 to 25.0% in those evaluating Datopotamab-Deruxtecan (TROP-2 target). Incidence of 9.9 and 5% of ILD was observed with Telisotuzumab-Vedotin (c-MET target) and Patritumab-Deruxtecan (Her-3 target), respectively. No cases of ILD have been reported with Sacituzumab-Govitecan (TROP-2 target) or Tusamitamab-Ravtansine (CEACAM5 target).
PERSPECTIVES:
Several risk factors for ADC-induced ILD seem to emerge, including respiratory comorbidities, renal insufficiency, or type and dosage of ADC. Current studies are focusing on the combination of ADC and immunotherapy, although there are few data now available on pulmonary toxicity profiles.
CONCLUSION:
Among the many ADCs being developed, several can cause ILD of varying grades and intensity. Knowledge of their risks, diagnostic and therapeutic modalities is required in order to quickly detect and treat ADC-induced ILD.
01 Jan 2025·Current medicinal chemistry
Review
Author: Ding, Ke ; Zhang, Jinwei ; Trinder, Amelia
HER3 (Human Epidermal Growth Factor Receptor 3) is frequently overexpressed
in various cancers, including non-small cell lung cancer (NSCLC), with a prevalence
of 83% in primary tumors. Its involvement in tumorigenesis and resistance to targeted
therapies makes HER3 a promising target for cancer treatment. Despite being initially
considered “undruggable” due to its lack of catalytic activity, significant progress
has been made in the development of anti-HER3 therapeutics. Monoclonal antibodies
such as lumretuzumab, seribantumab, and patritumab have shown potential in targeting
HER3 to overcome resistance to epidermal growth factor receptor (EGFR) tyrosine kinase
inhibitors (TKIs). Additionally, antibody-drug conjugates (ADCs) like HER3-DXd
(patritumab deruxtecan) are new drug candidates that have demonstrated selective delivery
of cytotoxic chemicals to NSCLC cells by exploiting HER3's widespread expression,
minimizing cytotoxicity. This review aims to evaluate the efficacy of current
HER3 therapeutics in development and their therapeutic potential in NSCLC, incorporating
evidence from clinical trials.
161
News (Medical) associated with Patritumab14 hours ago
More than 92% of patients treated with Daiichi Sankyo and AstraZeneca’s ENHERTU were free of invasive disease at three years
DESTINY-Breast05 presented in ESMO Presidential Symposium I alongside DESTINY-Breast11 reinforces potential for ENHERTU to become a foundational treatment in curative-intent early breast cancer setting
TOKYO & BASKING RIDGE, N.J.--(BUSINESS WIRE)--Positive results from the DESTINY-Breast05 phase 3 trial showed ENHERTU® (trastuzumab deruxtecan) demonstrated a highly statistically significant and clinically meaningful improvement in invasive disease-free survival (IDFS) compared to trastuzumab emtansine (T-DM1) as a post-neoadjuvant treatment (after surgery) in patients with HER2 positive early breast cancer with residual invasive disease in the breast and/or axillary lymph nodes after neoadjuvant treatment and a high risk of disease recurrence. Results were presented today (LBA1) alongside the results of DESTINY-Breast11 (291O) in Presidential Symposium I at the 2025 European Society for Medical Oncology (#ESMO25) Congress.
ENHERTU is a specifically engineered HER2 directed DXd antibody drug conjugate (ADC) discovered by Daiichi Sankyo (TSE: 4568) and being jointly developed and commercialized by Daiichi Sankyo and AstraZeneca (LSE/STO/NASDAQ: AZN).
In the primary endpoint analysis, ENHERTU significantly reduced the risk of invasive disease recurrence or death (invasive disease-free survival [IDFS]) by 53% (hazard ratio [HR]=0.47; 95% confidence interval [CI]: 0.34-0.66; p<0.0001) compared to T-DM1 as a post-neoadjuvant treatment. ENHERTU demonstrated a three-year IDFS rate of 92.4% (95% CI: 89.7-94.4) compared to 83.7% with T-DM1 (95% CI: 80.2-86.7). IDFS findings were consistent across all prespecified subgroups.
In the key secondary endpoint analysis of disease-free survival (DFS), ENHERTU also significantly reduced the risk of disease recurrence or death by 53% (HR=0.47; 95% CI: 0.34-0.66; p<0.0001) compared to T-DM1 as a post-neoadjuvant treatment. The three-year DFS rate for ENHERTU was 92.3% (95% CI: 89.5-94.3) compared to 83.5% with T-DM1 (95% CI: 79.9-86.4). Treatment with ENHERTU also reduced the risk of distant disease recurrence (distant recurrence-free interval [DRFI]) by 51% (HR=0.49; 95% CI: 0.34-0.71) and the risk of brain metastases (brain metastasis-free interval [BMFI]) by 36% (HR=0.64; 95% CI: 0.35-1.17) versus T-DM1. DRFI at three years was 93.9% with ENHERTU (95% CI: 91.4-95.7) compared to 86.1% with T-DM1 (95% CI: 82.5-89.1). BMFI at three years was 97.6% with ENHERTU (95% CI: 96.2-98.5) compared to 95.8% with T-DM1 (95% CI: 93.6-97.2).
Overall survival (OS) was not mature at the time of this planned interim analysis (2.9% maturity at data cut-off) and will be assessed in future analyses (HR=0.61; 95% CI: 0.34-1.10).
“For patients with residual disease after neoadjuvant treatment, the post-neoadjuvant setting represents a critical second opportunity to reduce recurrence risk, and in DESTINY-Breast05 ENHERTU reduced the risk of early recurrence or death by 53% compared to the current standard of T-DM1,” said Charles Geyer, MD, Chief Scientific Officer of the NSABP Foundation, Professor of Medicine at the UPMC Hillman Cancer Center in Pittsburgh, Pennsylvania and Principal Investigator for the trial. “These results, coupled with the safety data from the trial, are likely to transform clinical practice in the post-neoadjuvant setting for patients with high-risk disease, with the potential for ENHERTU to set a new standard of care.”
The safety profile of ENHERTU observed in DESTINY-Breast05 was consistent with its known profile with no new safety concerns identified. Grade 3 or higher treatment emergent adverse event (TEAE) rates were comparable between ENHERTU (50.6%) and T-DM1 (51.9%). The most common grade 3 or higher TEAEs occurring in 5% or more of patients treated with ENHERTU were decreased neutrophil count (15.5%), decreased white blood cell count (10.0%) and decreased platelet count (6.7%). Rates of interstitial lung disease (ILD) or pneumonitis were low in both arms with ILD events occurring in 9.6% of the ENHERTU arm and 1.6% of the T-DM1 arm. The majority of ILD or pneumonitis events were low grade (grade 1 [ENHERTU=16; 2.0%; T-DM1=8; 1.0%] or grade 2 [n=52; 6.5%; T-DM1=5; 0.6%]). There were no grade 3 or higher ILD or pneumonitis events for T-DM1. In the ENHERTU arm, there were seven grade 3 events (0.9%), zero grade 4 events and two grade 5 events (0.2%) as determined by an independent adjudication committee.
“The results of DESTINY-Breast05 demonstrate a clear benefit of ENHERTU over the current standard of care following surgery in patients with HER2 positive early breast cancer at high risk of recurrence after neoadjuvant treatment, improving their chance for sustained long-term outcomes,” said Ken Takeshita, MD, Global Head, R&D, Daiichi Sankyo. “These results, coupled with the results of DESTINY-Breast11, illustrate the continued promise of ENHERTU to move earlier in the breast cancer treatment paradigm where it can have the greatest impact on the lives of patients.”
“Progress in treating HER2 positive early breast cancer has been significant, yet managing patients at a higher-risk of recurrence remains challenging,” said Susan Galbraith, MBBChir, PhD, Executive Vice President, Oncology Hematology R&D, AstraZeneca. “These landmark data, alongside those from DESTINY-Breast11, underscore the potential of ENHERTU to become a foundational treatment in early-stage breast cancer, increasing the likelihood that more patients could be cured in this setting.”
All patients in the DESTINY-Breast05 trial had received prior neoadjuvant chemotherapy and HER2 targeted therapy and the majority of patients received either concurrent (ENHERTU=53.5%; T-DM1=58.8%) or sequential (ENHERTU=39.9%; T-DM1=34.1%) radiotherapy treatment. The majority of patients were pathologic node positive following neoadjuvant treatment (ENHERTU=80.7%; T-DM1=80.5%). As of the data cut-off date of July 2, 2025, the median study duration was 29.9 months in the ENHERTU arm (range: 0.3-53.4) and 29.7 months in the T-DM1 arm (range: 0.1-54.4).
Summary of DESTINY-Breast05 Interim Analysis Results
Efficacy Measure
ENHERTU (5.4 mg/kg) (n=818)
T-DM1 (n=817)
IDFS i,ii,iii,iv
HR (95% CI) = 0.47 (0.34-0.66); p<0.0001
3-year IDFS %
(95% CI)
92.4% (89.7-94.4)
83.7% (80.2-86.7)
Total IDFS events n (%)
51 (6.2)
102 (12.5)
Category of first IDFS event, niii
Distant recurrent CNS
Distant recurrence (non-CNS)
Local invasive recurrence
Regional recurrence
Contralateral invasive recurrence
Death without prior event
17
25
1
1
0
7
25
52
5
6
6
8
DFS i,ii,v
HR (95% CI) = 0.47; (0.34-0.66); p<0.0001
3-year DFS % (95% CI)
92.3% (89.5-94.3)
83.5% (79.9-86.4)
Total DFS events n (%)
52 (6.4)
103 (12.6)
DRFI ii,vi
Patients with events (recurrence), n (%)
42 (5.1)
81 (9.9)
3-year event-free rate, % (95% CI)
93.9 (91.4-95.7)
86.1 (82.5-89.1)
HR (95% CI) = 0.49 (0.34-0.71)
BMFIii,vii
Patients with events (recurrence), n (%)
17 (2.1)
26 (3.2)
3-year event-free rate, % (95% CI)
97.6 (96.2-98.5)
95.8 (93.6-97.2)
HR (95% CI) = 0.64 (0.35-1.17)
OSviii
Patients with events (deaths), n (%)
18 (2.2)
29 (3.5)
Survival at 3 years, % (95% CI)
97.4 (95.8-98.4)
95.7 (93.5-97.2)
HR (95% CI) = 0.61; (0.34-1.10)
BMFI, brain-metastasis-free interval; CI, confidence interval; CNS, central nervous system; DFS, disease-free survival; DRFI, distant recurrence-free interval; HR, hazard ratio; IDFS, invasive disease-free survival; OS, overall survival
i IDFS and DFS, the primary and key secondary efficacy endpoints, were statistically evaluated using the pre-specified hierarchical testing procedure.
ii Based on investigator assessment
iii IDFS is defined as the time from randomization until the date of first occurrence of one of the following events: recurrence of ipsilateral invasive breast tumor, recurrence of ipsilateral locoregional invasive breast cancer, contralateral invasive breast cancer, a distant disease recurrence or death from any cause
iv Patients who experienced multiple types of IDFS events within 61 days after their first event are reported in the category according to the following hierarchy: distant recurrence CNS, distant recurrence non-CNS, local invasive recurrence, regional recurrence, contralateral breast cancer, death without a previous event
v DFS is defined as the time between randomization and the date of first occurrence of an IDFS event per STEEP criteria, including second primary non-breast cancer event or contralateral or ipsilateral ductal carcinoma in situ (DCIS)
vi DRFI is defined as the time between randomization and the date of distant breast cancer recurrence
vii BMFI is defined as the time between randomization and the date of documentation of brain metastases or leptomeningeal disease
viii OS was 2.9% mature at interim analysis
DESTINY-Breast05 was conducted in collaboration with the National Surgical Adjuvant Breast and Bowel Project Foundation (NSABP), the German Breast Group (GBG), Arbeitsgemeinschaft Gynäkologische Onkologie (AGO-B) and SOLTI Breast Cancer Research Group.
About DESTINY-Breast05
DESTINY-Breast05 is a global, multicenter, randomized, open-label, phase 3 trial evaluating the efficacy and safety of ENHERTU (5.4 mg/kg) versus T-DM1 in patients with HER2 positive primary breast cancer with residual invasive disease in breast or axillary lymph nodes following neoadjuvant therapy and a high risk of recurrence. High risk of recurrence was defined as presentation with inoperable cancer (prior to neoadjuvant therapy) or pathologically positive axillary lymph nodes following neoadjuvant therapy.
The primary endpoint of DESTINY-Breast05 is investigator-assessed IDFS. IDFS is defined as the time from randomization until first recurrence, distant recurrence or death from any cause. The key secondary endpoint is investigator-assessed DFS. Other secondary endpoints include OS, DRFI, BMFI and safety.
DESTINY-Breast05 enrolled 1,635 patients in Asia, Europe, North America, Oceania and South America. For more information about the trial, visit ClinicalTrials.gov.
About Post Neoadjuvant HER2 Positive Early Breast Cancer
Breast cancer is the second most common cancer and one of the leading causes of cancer-related deaths worldwide.1 More than two million breast cancer cases were diagnosed in 2022, with more than 665,000 deaths globally.1
HER2 is a tyrosine kinase receptor growth-promoting protein expressed on the surface of many types of tumors including breast cancer.2 HER2 protein overexpression may occur as a result of HER2 gene amplification and is often associated with aggressive disease and poor prognosis in breast cancer.2 Approximately one in five cases of breast cancer are considered HER2 positive.3
For patients with HER2 positive early breast cancer, achieving pCR with neoadjuvant treatment is the earliest indicator of improved long-term survival.4 However, approximately half of patients who receive neoadjuvant treatment do not reach pCR and have poorer long-term outcomes, putting them at increased risk of disease recurrence.4,5,6,7,8,9
Despite receiving additional treatment with T-DM1 for residual disease in the post-neoadjuvant setting, approximately 20% of patients still experience invasive disease or death, with no reduction in the risk of central nervous system (CNS) recurrence.10,11 Once patients are diagnosed with metastatic disease, the five-year survival rate drops from nearly 90% to approximately 30%.12
Post-neoadjuvant therapy represents a key opportunity to minimize the risk of recurrence and prevent progression to metastatic disease for patients with residual disease. New treatment options are needed in the early breast cancer setting to help reduce the likelihood of disease progression and improve long-term outcomes for more patients.
About ENHERTU
ENHERTU (trastuzumab deruxtecan; fam-trastuzumab deruxtecan-nxki in the U.S. only) is a HER2 directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC Technology, ENHERTU is the lead ADC in the oncology portfolio of Daiichi Sankyo and the most advanced program in AstraZeneca’s ADC scientific platform. ENHERTU consists of a HER2 monoclonal antibody attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers.
ENHERTU (5.4 mg/kg) is approved in more than 85 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic HER2 positive (immunohistochemistry [IHC] 3+ or in-situ hybridization (ISH)+) breast cancer who have received a prior anti-HER2-based regimen, either in the metastatic setting or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within six months of completing therapy based on the results from the DESTINY-Breast03 trial.
ENHERTU (5.4 mg/kg) is approved in more than 85 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic HER2 low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior systemic therapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy based on the results from the DESTINY-Breast04 trial.
ENHERTU (5.4 mg/kg) is approved in more than 45 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic hormone receptor (HR) positive, HER2 low (IHC 1+ or IHC 2+/ ISH-) or HER2 ultralow (IHC 0 with membrane staining) breast cancer, as determined by a locally or regionally approved test, that have progressed on one or more endocrine therapies in the metastatic setting based on the results from the DESTINY-Breast06 trial.
ENHERTU (5.4 mg/kg) is approved in more than 60 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2 (ERBB2) mutations, as detected by a locally or regionally approved test, and who have received a prior systemic therapy based on the results from the DESTINY-Lung02 and/or DESTINY-Lung05 trials. Continued approval in China and the U.S. for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
ENHERTU (6.4 mg/kg) is approved in more than 70 countries/regions worldwide for the treatment of adult patients with locally advanced or metastatic HER2 positive (IHC 3+ or IHC 2+/ISH+) gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen based on the results from the DESTINY-Gastric01, DESTINY-Gastric02 and/or DESTINY-Gastric06 trials. Continued approval in China for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
ENHERTU (5.4 mg/kg) is approved in more than 10 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic HER2 positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options based on efficacy results from the DESTINY-PanTumor02, DESTINY-Lung01 and DESTINY-CRC02 trials. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
About the ENHERTU Clinical Development Program
A comprehensive global clinical development program is underway evaluating the efficacy and safety of ENHERTU as a monotherapy or in combination or sequentially with other cancer medicines across multiple HER2 targetable cancers.
About the Daiichi Sankyo and AstraZeneca Collaboration
Daiichi Sankyo and AstraZeneca entered into a global collaboration to jointly develop and commercialize ENHERTU in March 2019 and DATROWAY® in July 2020, except in Japan where Daiichi Sankyo maintains exclusive rights for each ADC. Daiichi Sankyo is responsible for the manufacturing and supply of ENHERTU and DATROWAY.
About the ADC Portfolio of Daiichi Sankyo
The Daiichi Sankyo ADC portfolio consists of seven ADCs in clinical development crafted from two distinct ADC technology platforms discovered in-house by Daiichi Sankyo.
The ADC platform furthest in clinical development is Daiichi Sankyo’s DXd ADC Technology where each ADC consists of a monoclonal antibody attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers. The DXd ADC portfolio currently consists of ENHERTU, a HER2 directed ADC, and DATROWAY, a TROP2 directed ADC, which are being jointly developed and commercialized globally with AstraZeneca. Patritumab deruxtecan (HER3-DXd), a HER3 directed ADC, ifinatamab deruxtecan (I-DXd), a B7-H3 directed ADC, and raludotatug deruxtecan (R-DXd), a CDH6 directed ADC, are being jointly developed and commercialized globally with Merck & Co., Inc, Rahway, NJ, USA. DS-3939, a TA-MUC1 directed ADC, is being developed by Daiichi Sankyo.
The second Daiichi Sankyo ADC platform consists of a monoclonal antibody attached to a modified pyrrolobenzodiazepine (PBD) payload. DS-9606, a CLDN6 directed PBD ADC, is the first of several planned ADCs in clinical development utilizing this platform.
Ifinatamab deruxtecan, patritumab deruxtecan, raludotatug deruxtecan, DS-3939 and DS-9606 are investigational medicines that have not been approved for any indication in any country. Safety and efficacy have not been established.
ENHERTU U.S. Important Safety Information
Indications
ENHERTU is a HER2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
Unresectable or metastatic HER2-positive (IHC 3+ or ISH positive) breast cancer who have received a prior anti-HER2-based regimen either: In the metastatic setting, or In the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy
In the metastatic setting, or
In the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy
Unresectable or metastatic: Hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer, as determined by an FDA-approved test, that has progressed on one or more endocrine therapies in the metastatic setting HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy
Hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer, as determined by an FDA-approved test, that has progressed on one or more endocrine therapies in the metastatic setting
HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy
Unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy This indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Locally advanced or metastatic HER2-positive (IHC 3+ or IHC 2+/ISH positive) gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen
Unresectable or metastatic HER2-positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options This indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
WARNING: INTERSTITIAL LUNG DISEASE and EMBRYO-FETAL TOXICITY
Contraindications
None.
Warnings and Precautions
Interstitial Lung Disease / Pneumonitis
Severe, life-threatening, or fatal interstitial lung disease (ILD), including pneumonitis, can occur in patients treated with ENHERTU. A higher incidence of Grade 1 and 2 ILD/pneumonitis has been observed in patients with moderate renal impairment. Advise patients to immediately report cough, dyspnea, fever, and/or any new or worsening respiratory symptoms. Monitor patients for signs and symptoms of ILD. Promptly investigate evidence of ILD. Evaluate patients with suspected ILD by radiographic imaging. Consider consultation with a pulmonologist. For asymptomatic ILD/pneumonitis (Grade 1), interrupt ENHERTU until resolved to Grade 0, then if resolved in ≤28 days from date of onset, maintain dose. If resolved in >28 days from date of onset, reduce dose 1 level. Consider corticosteroid treatment as soon as ILD/pneumonitis is suspected (e.g., ≥0.5 mg/kg/day prednisolone or equivalent). For symptomatic ILD/pneumonitis (Grade 2 or greater), permanently discontinue ENHERTU. Promptly initiate systemic corticosteroid treatment as soon as ILD/pneumonitis is suspected (e.g., ≥1 mg/kg/day prednisolone or equivalent) and continue for at least 14 days followed by gradual taper for at least 4 weeks.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, ILD occurred in 12% of patients. Median time to first onset was 5.5 months (range: 0.9 to 31.5). Fatal outcomes due to ILD and/or pneumonitis occurred in 0.9% of patients treated with ENHERTU.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, ILD occurred in 10% of patients. Median time to first onset was 2.8 months (range: 1.2 to 21).
Neutropenia
Severe neutropenia, including febrile neutropenia, can occur in patients treated with ENHERTU. Monitor complete blood counts prior to initiation of ENHERTU and prior to each dose, and as clinically indicated. For Grade 3 neutropenia (Absolute Neutrophil Count [ANC] <1.0 to 0.5 x 109/L), interrupt ENHERTU until resolved to Grade 2 or less, then maintain dose. For Grade 4 neutropenia (ANC <0.5 x 109/L), interrupt ENHERTU until resolved to Grade 2 or less, then reduce dose by 1 level. For febrile neutropenia (ANC <1.0 x 109/L and temperature >38.3º C or a sustained temperature of ≥38º C for more than 1 hour), interrupt ENHERTU until resolved, then reduce dose by 1 level.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, a decrease in neutrophil count was reported in 65% of patients. Nineteen percent had Grade 3 or 4 decreased neutrophil count. Median time to first onset of decreased neutrophil count was 22 days (range: 2 to 939). Febrile neutropenia was reported in 1.2% of patients.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, a decrease in neutrophil count was reported in 72% of patients. Fifty-one percent had Grade 3 or 4 decreased neutrophil count. Median time to first onset of decreased neutrophil count was 16 days (range: 4 to 187). Febrile neutropenia was reported in 4.8% of patients.
Left Ventricular Dysfunction
Patients treated with ENHERTU may be at increased risk of developing left ventricular dysfunction. Left ventricular ejection fraction (LVEF) decrease has been observed with anti-HER2 therapies, including ENHERTU. Assess LVEF prior to initiation of ENHERTU and at regular intervals during treatment as clinically indicated. Manage LVEF decrease through treatment interruption. When LVEF is >45% and absolute decrease from baseline is 10-20%, continue treatment with ENHERTU. When LVEF is 40-45% and absolute decrease from baseline is 20%, interrupt ENHERTU and repeat LVEF assessment within 3 weeks. If LVEF of 20% is confirmed, permanently discontinue ENHERTU. Permanently discontinue ENHERTU in patients with symptomatic congestive heart failure. Treatment with ENHERTU has not been studied in patients with a history of clinically significant cardiac disease or LVEF <50% prior to initiation of treatment.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, LVEF decrease was reported in 4.6% of patients, of which 0.6% were Grade 3 or 4.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, no clinical adverse events of heart failure were reported; however, on echocardiography, 8% were found to have asymptomatic Grade 2 decrease in LVEF.
Embryo-Fetal Toxicity
ENHERTU can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risks to a fetus. Verify the pregnancy status of females of reproductive potential prior to the initiation of ENHERTU. Advise females of reproductive potential to use effective contraception during treatment and for 7 months after the last dose of ENHERTU. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose of ENHERTU.
Additional Dose Modifications
Thrombocytopenia
For Grade 3 thrombocytopenia (platelets <50 to 25 x 109/L) interrupt ENHERTU until resolved to Grade 1 or less, then maintain dose. For Grade 4 thrombocytopenia (platelets <25 x 109/L) interrupt ENHERTU until resolved to Grade 1 or less, then reduce dose by 1 level.
Adverse Reactions
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
The pooled safety population reflects exposure to ENHERTU 5.4 mg/kg intravenously every 3 weeks in 2233 patients in Study DS8201-A-J101 (NCT02564900), DESTINY-Breast01, DESTINY-Breast02, DESTINY-Breast03, DESTINY-Breast04, DESTINY-Breast06, DESTINY-Lung01, DESTINY-Lung02, DESTINY-CRC02, and DESTINY-PanTumor02. Among these patients, 67% were exposed for >6 months and 38% were exposed for >1 year. In this pooled safety population, the most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count (73%), nausea (72%), decreased hemoglobin (67%), decreased neutrophil count (65%), decreased lymphocyte count (60%), fatigue (55%), decreased platelet count (48%), increased aspartate aminotransferase (46%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (39%), vomiting (38%), alopecia (37%), constipation (32%), decreased blood potassium (32%), decreased appetite (31%), diarrhea (30%), and musculoskeletal pain (24%).
HER2-Positive Metastatic Breast Cancer
DESTINY-Breast03
The safety of ENHERTU was evaluated in 257 patients with unresectable or metastatic HER2-positive breast cancer who received at least 1 dose of ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast03. The median duration of treatment was 14 months (range: 0.7 to 30) for patients who received ENHERTU.
Serious adverse reactions occurred in 19% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were vomiting, ILD, pneumonia, pyrexia, and urinary tract infection. Fatalities due to adverse reactions occurred in 0.8% of patients including COVID-19 and sudden death (1 patient each).
ENHERTU was permanently discontinued in 14% of patients, of which ILD/pneumonitis accounted for 8%. Dose interruptions due to adverse reactions occurred in 44% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, leukopenia, anemia, thrombocytopenia, pneumonia, nausea, fatigue, and ILD/pneumonitis. Dose reductions occurred in 21% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were nausea, neutropenia, and fatigue.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea (76%), decreased white blood cell count (74%), decreased neutrophil count (70%), increased aspartate aminotransferase (67%), decreased hemoglobin (64%), decreased lymphocyte count (55%), increased alanine aminotransferase (53%), decreased platelet count (52%), fatigue (49%), vomiting (49%), increased blood alkaline phosphatase (49%), alopecia (37%), decreased blood potassium (35%), constipation (34%), musculoskeletal pain (31%), diarrhea (29%), decreased appetite (29%), headache (22%), respiratory infection (22%), abdominal pain (21%), increased blood bilirubin (20%), and stomatitis (20%).
HER2-Low and HER2-Ultralow Metastatic Breast Cancer
DESTINY-Breast06
The safety of ENHERTU was evaluated in 434 patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast06. The median duration of treatment was 11 months (range: 0.4 to 39.6) for patients who received ENHERTU.
Serious adverse reactions occurred in 20% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, COVID-19, febrile neutropenia, and hypokalemia. Fatalities due to adverse reactions occurred in 2.8% of patients including ILD (0.7%); sepsis (0.5%); and COVID-19 pneumonia, bacterial meningoencephalitis, neutropenic sepsis, peritonitis, cerebrovascular accident, general physical health deterioration (0.2% each).
ENHERTU was permanently discontinued in 14% of patients. The most frequent adverse reaction (>2%) associated with permanent discontinuation was ILD/pneumonitis. Dose interruptions due to adverse reactions occurred in 48% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were COVID-19, decreased neutrophil count, anemia, pyrexia, pneumonia, decreased white blood cell count, and ILD. Dose reductions occurred in 25% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were nausea, fatigue, decreased platelet count, and decreased neutrophil count.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count (86%), decreased neutrophil count (75%), nausea (70%), decreased hemoglobin (69%), decreased lymphocyte count (66%), fatigue (53%), decreased platelet count (48%), alopecia (48%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (43%), increased aspartate aminotransferase (41%), decreased blood potassium (35%), diarrhea (34%), vomiting (34%), constipation (32%), decreased appetite (26%), COVID-19 (26%), and musculoskeletal pain (24%).
DESTINY-Breast04
The safety of ENHERTU was evaluated in 371 patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast04. The median duration of treatment was 8 months (range: 0.2 to 33) for patients who received ENHERTU.
Serious adverse reactions occurred in 28% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, pneumonia, dyspnea, musculoskeletal pain, sepsis, anemia, febrile neutropenia, hypercalcemia, nausea, pyrexia, and vomiting. Fatalities due to adverse reactions occurred in 4% of patients including ILD/pneumonitis (3 patients); sepsis (2 patients); and ischemic colitis, disseminated intravascular coagulation, dyspnea, febrile neutropenia, general physical health deterioration, pleural effusion, and respiratory failure (1 patient each).
ENHERTU was permanently discontinued in 16% of patients, of which ILD/pneumonitis accounted for 8%. Dose interruptions due to adverse reactions occurred in 39% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, fatigue, anemia, leukopenia, COVID-19, ILD/pneumonitis, increased transaminases, and hyperbilirubinemia. Dose reductions occurred in 23% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, thrombocytopenia, and neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea (76%), decreased white blood cell count (70%), decreased hemoglobin (64%), decreased neutrophil count (64%), decreased lymphocyte count (55%), fatigue (54%), decreased platelet count (44%), alopecia (40%), vomiting (40%), increased aspartate aminotransferase (38%), increased alanine aminotransferase (36%), constipation (34%), increased blood alkaline phosphatase (34%), decreased appetite (32%), musculoskeletal pain (32%), diarrhea (27%), and decreased blood potassium (25%).
HER2-Mutant Unresectable or Metastatic NSCLC (5.4 mg/kg)
DESTINY-Lung02 evaluated 2 dose levels (5.4 mg/kg [n=101] and 6.4 mg/kg [n=50]); however, only the results for the recommended dose of 5.4 mg/kg intravenously every 3 weeks are described below due to increased toxicity observed with the higher dose in patients with NSCLC, including ILD/pneumonitis.
The safety of ENHERTU was evaluated in 101 patients with HER2-mutant unresectable or metastatic NSCLC who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks until disease progression or unacceptable toxicity in DESTINY-Lung02. Nineteen percent of patients were exposed for >6 months.
Serious adverse reactions occurred in 30% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, thrombocytopenia, dyspnea, nausea, pleural effusion, and increased troponin I. Fatality occurred in 1 patient with suspected ILD/pneumonitis (1%).
ENHERTU was permanently discontinued in 8% of patients. Adverse reactions which resulted in permanent discontinuation of ENHERTU were ILD/pneumonitis, diarrhea, decreased blood potassium, hypomagnesemia, myocarditis, and vomiting. Dose interruptions of ENHERTU due to adverse reactions occurred in 23% of patients. Adverse reactions which required dose interruption (>2%) included neutropenia and ILD/pneumonitis. Dose reductions due to an adverse reaction occurred in 11% of patients.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea (61%), decreased white blood cell count (60%), decreased hemoglobin (58%), decreased neutrophil count (52%), decreased lymphocyte count (43%), decreased platelet count (40%), decreased albumin (39%), increased aspartate aminotransferase (35%), increased alanine aminotransferase (34%), fatigue (32%), constipation (31%), decreased appetite (30%), vomiting (26%), increased alkaline phosphatase (22%), and alopecia (21%).
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
The safety of ENHERTU was evaluated in 187 patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma in DESTINY-Gastric01. Patients intravenously received at least 1 dose of either ENHERTU (N=125) 6.4 mg/kg every 3 weeks or either irinotecan (N=55) 150 mg/m2 biweekly or paclitaxel (N=7) 80 mg/m2 weekly for 3 weeks. The median duration of treatment was 4.6 months (range: 0.7 to 22.3) for patients who received ENHERTU.
Serious adverse reactions occurred in 44% of patients receiving ENHERTU 6.4 mg/kg. Serious adverse reactions in >2% of patients who received ENHERTU were decreased appetite, ILD, anemia, dehydration, pneumonia, cholestatic jaundice, pyrexia, and tumor hemorrhage. Fatalities due to adverse reactions occurred in 2.4% of patients: disseminated intravascular coagulation, large intestine perforation, and pneumonia occurred in 1 patient each (0.8%).
ENHERTU was permanently discontinued in 15% of patients, of which ILD accounted for 6%. Dose interruptions due to adverse reactions occurred in 62% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, anemia, decreased appetite, leukopenia, fatigue, thrombocytopenia, ILD, pneumonia, lymphopenia, upper respiratory tract infection, diarrhea, and decreased blood potassium. Dose reductions occurred in 32% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were neutropenia, decreased appetite, fatigue, nausea, and febrile neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased hemoglobin (75%), decreased white blood cell count (74%), decreased neutrophil count (72%), decreased lymphocyte count (70%), decreased platelet count (68%), nausea (63%), decreased appetite (60%), increased aspartate aminotransferase (58%), fatigue (55%), increased blood alkaline phosphatase (54%), increased alanine aminotransferase (47%), diarrhea (32%), decreased blood potassium (30%), vomiting (26%), constipation (24%), increased blood bilirubin (24%), pyrexia (24%), and alopecia (22%).
HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
The safety of ENHERTU was evaluated in 347 adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast01, DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02. The median duration of treatment was 8.3 months (range 0.7 to 30.2).
Serious adverse reactions occurred in 34% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were sepsis, pneumonia, vomiting, urinary tract infection, abdominal pain, nausea, pneumonitis, pleural effusion, hemorrhage, COVID-19, fatigue, acute kidney injury, anemia, cellulitis, and dyspnea. Fatalities due to adverse reactions occurred in 6.3% of patients including ILD/pneumonitis (2.3%), cardiac arrest (0.6%), COVID-19 (0.6%), and sepsis (0.6%). The following events occurred in 1 patient each (0.3%): acute kidney injury, cerebrovascular accident, general physical health deterioration, pneumonia, and hemorrhagic shock.
ENHERTU was permanently discontinued in 15% of patients, of which ILD/pneumonitis accounted for 10%. Dose interruptions due to adverse reactions occurred in 48% of patients. The most frequent adverse reactions (>2%) associated with dose interruption were decreased neutrophil count, anemia, COVID-19, fatigue, decreased white blood cell count, and ILD/pneumonitis. Dose reductions occurred in 27% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, decreased neutrophil count, ILD/pneumonitis, and diarrhea.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count (75%), nausea (69%), decreased hemoglobin (67%), decreased neutrophil count (66%), fatigue (59%), decreased lymphocyte count (58%), decreased platelet count (51%), increased aspartate aminotransferase (45%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (36%), vomiting (35%), decreased appetite (34%), alopecia (34%), diarrhea (31%), decreased blood potassium (29%), constipation (28%), decreased sodium (22%), stomatitis (20%), and upper respiratory tract infection (20%).
Use in Specific Populations
Pregnancy: ENHERTU can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risks to a fetus. There are clinical considerations if ENHERTU is used in pregnant women, or if a patient becomes pregnant within 7 months after the last dose of ENHERTU.
Lactation: There are no data regarding the presence of ENHERTU in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with ENHERTU and for 7 months after the last dose.
Females and Males of Reproductive Potential: Pregnancy testing : Verify pregnancy status of females of reproductive potential prior to initiation of ENHERTU. Contraception : Females : ENHERTU can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with ENHERTU and for 7 months after the last dose. Males : Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose. Infertility : ENHERTU may impair male reproductive function and fertility.
Pediatric Use: Safety and effectiveness of ENHERTU have not been established in pediatric patients.
Geriatric Use: Of the 1741 patients with HER2-positive, HER2-low, or HER2-ultralow breast cancer treated with ENHERTU 5.4 mg/kg, 24% were ≥65 years and 4.9% were ≥75 years. No overall differences in efficacy within clinical studies were observed between patients ≥65 years of age compared to younger patients. There was a higher incidence of Grade 3-4 adverse reactions observed in patients aged ≥65 years (61%) as compared to younger patients (52%). Of the 101 patients with HER2-mutant unresectable or metastatic NSCLC treated with ENHERTU 5.4 mg/kg, 40% were ≥65 years and 8% were ≥75 years. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients. Of the 125 patients with HER2-positive locally advanced or metastatic gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg in DESTINY-Gastric01, 56% were ≥65 years and 14% were ≥75 years. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients. Of the 192 patients with HER2-positive (IHC 3+) unresectable or metastatic solid tumors treated with ENHERTU 5.4 mg/kg in DESTINY-PanTumor02, DESTINY-Lung01, or DESTINY-CRC02, 39% were ≥65 years and 9% were ≥75 years. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients.
Renal Impairment: A higher incidence of Grade 1 and 2 ILD/pneumonitis has been observed in patients with moderate renal impairment. Monitor patients with moderate renal impairment more frequently. The recommended dosage of ENHERTU has not been established for patients with severe renal impairment (CLcr <30 mL/min).
Hepatic Impairment: In patients with moderate hepatic impairment, due to potentially increased exposure, closely monitor for increased toxicities related to the topoisomerase inhibitor, DXd. The recommended dosage of ENHERTU has not been established for patients with severe hepatic impairment (total bilirubin >3 times ULN and any AST).
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or fda.gov/medwatch.
Please see accompanying full Prescribing Information, including Boxed WARNINGS, and Medication Guide.
About Daiichi Sankyo
Daiichi Sankyo is an innovative global healthcare company contributing to the sustainable development of society that discovers, develops and delivers new standards of care to enrich the quality of life around the world. With more than 120 years of experience, Daiichi Sankyo leverages its world-class science and technology to create new modalities and innovative medicines for people with cancer, cardiovascular and other diseases with high unmet medical need. For more information, please visit www.daiichisankyo.com.
References
1 World Health Organization. Breast Fact Sheet. Accessed October 2025.
2 Cheng X, et al. Genes. 2024;15(7):903.
3 Tarantino P, et al. J An Onc. 2023;34(8):645-659.
4 Spring LM, et al. Clin Cancer Res. 2020;26(12):2838-2284.
5 Schneeweiss A, et al. Annals of Oncol. 2013; 24:2278-2284.
6 Swain S, et al. Annals of Oncol. 2018; 29:646-653.
7 Huober J, et al. J Clin Oncol. 2022; 40:2946-2956.
8 Masuda N, et al. Breast Cancer Res Treat. 2020; 180:135-146.
9 Gao H, et al. Presented ASCO Annual Meeting 2025.
10 Geyer C, et al. N Engl J Med. 2025; 392(3):249-257.
11 NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version 4.2025.
12 National Cancer Institute. SEER Cancer Stat Facts: Female Breast Cancer. Accessed October 2025.
Clinical ResultDrug ApprovalPhase 3ADCAccelerated Approval
14 hours ago
Highest reported pathologic complete response rate seen in a phase 3 registrational trial for HER2 positive early breast cancer with favorable safety profile versus standard treatment
DESTINY-Breast11 presented in ESMO Presidential Symposium I alongside DESTINY-Breast05 reinforces potential for Daiichi Sankyo and AstraZeneca’s ENHERTU to become a foundational treatment in curative-intent early breast cancer setting
TOKYO & BASKING RIDGE, N.J.--(BUSINESS WIRE)--Positive results from the DESTINY-Breast11 phase 3 trial showed ENHERTU® (trastuzumab deruxtecan) followed by paclitaxel, trastuzumab and pertuzumab (THP) in the neoadjuvant setting (before surgery) demonstrated a statistically significant and clinically meaningful improvement in the pathologic complete response (pCR) rate when compared with dose-dense doxorubicin and cyclophosphamide followed by THP (ddAC-THP) in patients with high-risk, locally advanced HER2 positive early-stage breast cancer. Pathologic complete response is defined as no evidence of invasive cancer cells in the removed breast tissue and lymph nodes following treatment. Results were presented today (291O) alongside the results from the DESTINY-Breast05 (LBA1) phase 3 trial during Presidential Symposium I at the 2025 European Society for Medical Oncology (#ESMO25) Congress.
ENHERTU is a specifically engineered HER2 directed DXd antibody drug conjugate (ADC) discovered by Daiichi Sankyo (TSE: 4568) and being jointly developed and commercialized by Daiichi Sankyo and AstraZeneca (LSE/STO/NASDAQ: AZN).
In the trial, ENHERTU followed by THP resulted in a pCR rate of 67.3% compared to 56.3% with ddAC-THP, representing a pCR rate improvement of 11.2% (95% confidence interval [CI]: 4.0-18.3; p=0.003). Improvements in pCR rates were observed across both HR positive and HR negative subgroups. After surgery, 81.3% of patients who received neoadjuvant therapy in the ENHERTU followed by THP arm had no or minimal residual invasive cancer (Residual Cancer Burden [RCB] 0+I) detected in the resected breast or lymph node tissue compared to 69.1% of patients in the comparator arm.
The secondary endpoint of event-free survival (EFS) was not mature at the time of this analysis (4.5% maturity at data cut-off); however, an early analysis showed a trend favoring ENHERTU followed by THP versus ddAC-THP (HR=0.56; 95% CI: 0.26-1.17).
“For patients with early breast cancer who are at high risk of disease recurrence, using the most effective treatment option at the earliest opportunity is critical to prevent recurrence, optimize safety and improve the potential for cure,” said Nadia Harbeck, Director of Breast Center, Cancer Department of OB&GYN and CCC Munich, LMU University Hospital, Germany and Principal Investigator for the trial. “In the DESTINY-Breast11 trial, more than two-thirds of patients had a pathologic complete response with trastuzumab deruxtecan followed by THP, suggesting a potential new standard of care in the neoadjuvant setting for patients with high-risk, HER2 positive early breast cancer.”
The safety profile of ENHERTU followed by THP in DESTINY-Breast11 was consistent with the known profiles of each individual therapy with no new safety concerns identified. ENHERTU followed by THP showed a favorable safety profile compared to ddAC-THP with reduced rates of grade 3 or higher treatment emergent adverse events (TEAEs) (ENHERTU followed by THP=37.5%; ddAC-THP=55.8%), serious adverse events (AEs) (ENHERTU followed by THP=10.6%; ddAC-THP=20.2%), treatment interruptions (ENHERTU followed by THP=37.8%; ddAC-THP=54.5%) and left ventricular dysfunction (ENHERTU followed by THP=1.3%; ddAC-THP=6.1%). The most common grade 3 or higher TEAEs occurring in 5% or more of patients treated with ENHERTU followed by THP were neutropenia (13.8%), diarrhea (5.9%) and increased transaminases (5.0%). Rates of interstitial lung disease (ILD) or pneumonitis were low and similar between arms with ILD events occurring in 4.4% (n=14/320) of patients treated with ENHERTU followed by THP compared to 5.1% (n=16/312) in those treated with ddAC-THP. The majority of ILD or pneumonitis events were low grade (grade 1 or grade 2 [ENHERTU followed by THP=12; ddAC-THP=10]), with one grade 3 or grade 4 events in the ENHERTU followed by THP arm and five grade 3 or 4 events in the ddAC-THP arm. There was one grade 5 ILD event in each arm as determined by an independent adjudication committee.
“While achieving a pathologic complete response in HER2 positive early-stage breast cancer is critical for reducing disease recurrence and improving long-term prognosis, approximately half of patients still show evidence of residual disease following surgery with currently available neoadjuvant treatment options,” said Ken Takeshita, MD, Global Head, R&D, Daiichi Sankyo. “The results from DESTINY-Breast11 show that treatment with ENHERTU followed by THP prior to surgery resulted in no evidence of residual invasive disease in two-thirds of patients, illustrating the first treatment regimen in more than a decade to significantly improve outcomes in the earliest treatment setting for HER2 positive breast cancer.”
“The goal of treatment in the early breast cancer setting is to provide patients with the best possible chance for cure while optimizing the tolerability of the treatment regimen,” said Susan Galbraith, MBBChir, PhD, Executive Vice President, Oncology Hematology R&D, AstraZeneca. “The impressive pathologic response rates and favorable safety profile seen with ENHERTU followed by THP in DESTINY-Breast11 have the potential to transform treatment in the neoadjuvant setting and underscore the importance of bringing ENHERTU into earlier stages of HER2 positive disease.”
Approximately half of patients in DESTINY-Breast11 had a primary clinical tumor stage of 0-2 (cT0-2; ENHERTU followed by THP=54.8%; ddAC-THP=58.8%) and nearly half had a primary clinical tumor stage of 3-4 (cT3-4; ENHERTU followed by THP=45.2%; ddAC-THP=41.3%). The majority of patients were node positive (ENHERTU followed by THP=89.4%; ddAC-THP=87.8%) and had an ECOG PS (Eastern Cooperative Oncology Group performance status) of 0 (ENHERTU followed by THP=86.6%; ddAC-THP=87.5%). Nearly all patients underwent surgery following neoadjuvant treatment (ENHERTU followed by THP=97.2%; ddAC-THP=93.8%). As of the data cut-off date of March 12, 2025, the median duration of follow-up was 24.3 months in the ENHERTU followed by THP arm and 23.6 months in the ddAC-THP arm.
Summary of DESTINY-Breast11 Primary Results
Efficacy Measure
ENHERTU (5.4 mg/kg; 4 cycles) followed by THP (4 cycles) (n=321)
ddAC-THP
(4 cycles) (n=320)
pCRi
pCR rate %ii
67.3%
56.3%
ΔpCR % (95% CI)ii,iii
11.2% (4.0-18.3)
p=0.003
Hormone receptor positive subgroup pCR rate %ii
61.4%
52.3%
ΔpCR % (95% CI)ii,iii
9.1% (0.2-17.9)
Hormone receptor negative subgroup pCR rate %ii
83.1%
67.1%
ΔpCR % (95% CI)ii,iii
16.1% (3.0-28.8)
RCB (0+I)i,iv
RCB (0+I) rate %
81.3%
69.1%
ΔRCB %
12.2%
RCB-I rate %
68.8%
57.5%
RCB-0 rate %
12.5%
11.6%
Hormone receptor positive subgroup RCB (0+I) rate %
78.0%
64.7%
ΔRCB % (95% CI)
13.3%
Hormone receptor positive RCB-I rate %
63.1%
52.8%
Hormone receptor positive RCB-0 rate %
14.8%
11.9%
Hormone receptor negative subgroup RCB (0+I) rate %
90.4%
81.2%
ΔRCB % (95% CI)
9.2%
Hormone receptor negative RCB-I rate %
84.3%
70.6%
Hormone receptor negative RCB-0 rate %
6.0%
10.6%
EFSi,v,vi
2-year EFS %
(95% CI)
96.9% (95% CI 93.5-98.6)
93.1% (88.7-95.8)
HR=0.56 (0.26, 1.17)
CI, confidence interval; EFS, event-free survival; HR, hazard ratio; pCR, pathologic complete response; RCB (0+I), residual cancer burden
i Based on blinded central review
ii pCR responders were defined as patients who only received randomized study treatment (at least one dose) and had pCR
iii Stratified Miettinen & Nurminen method; p value crossed the 0.03 prespecified boundary
iv RCB is based on raw data and is not corrected for non-starters, or any bridging/off study neoadjuvant treatment; therefore, there may be differences between pCR and RCB-0
v EFS was 4.5% mature at interim analysis
vi Median duration of follow up was 24.3 months with ENHERTU followed by THP and 23.6 months with ddAC-THP
The ENHERTU monotherapy arm (n=286) was closed early following a recommendation from the Independent Data Monitoring Committee (IDMC). The recommendation was based on multiple factors including a lower pCR rate, low likelihood that ENHERTU alone would be superior to ddAC-THP, and the timing of surgery. The recommendation was not related to safety.
A supplemental Biologics License Application for ENHERTU followed by THP based on data from DESTINY-Breast11 is currently under review by the U.S. Food and Drug Administration (FDA).
About DESTINY-Breast11
DESTINY-Breast11 is a global, multicenter, randomized, open-label, phase 3 trial evaluating the efficacy and safety of neoadjuvant ENHERTU (5.4 mg/kg) monotherapy or ENHERTU followed by THP (paclitaxel, trastuzumab and pertuzumab) versus ddAC-THP in patients with high-risk (lymph node positive [N1-3] or primary tumor stage T3-4), locally advanced or inflammatory HER2 positive early-stage breast cancer.
Patients were randomized 1:1:1 to receive either eight cycles of ENHERTU monotherapy; four cycles of ENHERTU followed by four cycles of THP; or four cycles of ddAC followed by four cycles of THP.
The primary endpoint of DESTINY-Breast11 is rate of pCR (absence of invasive disease in the breast and lymph nodes). Secondary endpoints include EFS, invasive disease-free survival, overall survival and safety.
DESTINY-Breast11 enrolled 927 patients across multiple sites in Asia, Europe, North America and South America. For more information about the trial, visit ClinicalTrials.gov.
About Neoadjuvant HER2 Positive Early Breast Cancer
Breast cancer is the second most common cancer and one of the leading causes of cancer-related deaths worldwide.1 More than two million breast cancer cases were diagnosed in 2022, with more than 665,000 deaths globally.1
HER2 is a tyrosine kinase receptor growth-promoting protein expressed on the surface of many types of tumors, including breast cancer.2 HER2 protein overexpression may occur as a result of HER2 gene amplification and is often associated with aggressive disease and poor prognosis in breast cancer.2 Approximately one in five cases of breast cancer are considered HER2 positive.3
Approximately one in three patients with HER2 positive early-stage breast cancer are considered high-risk, meaning they are more likely to experience disease recurrence and have a poor prognosis.4 For patients with HER2 positive early breast cancer, achieving pCR with neoadjuvant treatment is the earliest indicator of improved long-term survival.5 However, approximately half of patients who receive neoadjuvant treatment do not reach pCR, putting them at increased risk of disease recurrence.5,6,7,8,9,10
The current standard of care in the HER2 positive neoadjuvant setting in many regions of the world consists of combination chemotherapy regimens.11 These regimens often include anthracyclines, which can be challenging for patients to tolerate and may result in long-term cardiotoxicity, reinforcing the need for new treatment options.11,12,13
About ENHERTU
ENHERTU (trastuzumab deruxtecan; fam-trastuzumab deruxtecan-nxki in the U.S. only) is a HER2 directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC Technology, ENHERTU is the lead ADC in the oncology portfolio of Daiichi Sankyo and the most advanced program in AstraZeneca’s ADC scientific platform. ENHERTU consists of a HER2 monoclonal antibody attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers.
ENHERTU (5.4 mg/kg) is approved in more than 85 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic HER2 positive (immunohistochemistry [IHC] 3+ or in-situ hybridization (ISH)+) breast cancer who have received a prior anti-HER2-based regimen, either in the metastatic setting or in the neoadjuvant or adjuvant setting, and have developed disease recurrence during or within six months of completing therapy based on the results from the DESTINY-Breast03 trial.
ENHERTU (5.4 mg/kg) is approved in more than 85 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic HER2 low (IHC 1+ or IHC 2+/ISH-) breast cancer who have received a prior systemic therapy in the metastatic setting or developed disease recurrence during or within six months of completing adjuvant chemotherapy based on the results from the DESTINY-Breast04 trial.
ENHERTU (5.4 mg/kg) is approved in more than 45 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic hormone receptor (HR) positive, HER2 low (IHC 1+ or IHC 2+/ ISH-) or HER2 ultralow (IHC 0 with membrane staining) breast cancer, as determined by a locally or regionally approved test, that have progressed on one or more endocrine therapies in the metastatic setting based on the results from the DESTINY-Breast06 trial.
ENHERTU (5.4 mg/kg) is approved in more than 60 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2 (ERBB2) mutations, as detected by a locally or regionally approved test, and who have received a prior systemic therapy based on the results from the DESTINY-Lung02 and/or DESTINY-Lung05 trials. Continued approval in China and the U.S. for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
ENHERTU (6.4 mg/kg) is approved in more than 70 countries/regions worldwide for the treatment of adult patients with locally advanced or metastatic HER2 positive (IHC 3+ or IHC 2+/ISH+) gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen based on the results from the DESTINY-Gastric01, DESTINY-Gastric02 and/or DESTINY-Gastric06 trials. Continued approval in China for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
ENHERTU (5.4 mg/kg) is approved in more than 10 countries/regions worldwide for the treatment of adult patients with unresectable or metastatic HER2 positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options based on efficacy results from the DESTINY-PanTumor02, DESTINY-Lung01 and DESTINY-CRC02 trials. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
About the ENHERTU Clinical Development Program
A comprehensive global clinical development program is underway evaluating the efficacy and safety of ENHERTU as a monotherapy or in combination or sequentially with other cancer medicines across multiple HER2 targetable cancers.
About the Daiichi Sankyo and AstraZeneca Collaboration
Daiichi Sankyo and AstraZeneca entered into a global collaboration to jointly develop and commercialize ENHERTU in March 2019 and DATROWAY® in July 2020, except in Japan where Daiichi Sankyo maintains exclusive rights for each ADC. Daiichi Sankyo is responsible for the manufacturing and supply of ENHERTU and DATROWAY.
About the ADC Portfolio of Daiichi Sankyo
The Daiichi Sankyo ADC portfolio consists of seven ADCs in clinical development crafted from two distinct ADC technology platforms discovered in-house by Daiichi Sankyo.
The ADC platform furthest in clinical development is Daiichi Sankyo’s DXd ADC Technology where each ADC consists of a monoclonal antibody attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers. The DXd ADC portfolio currently consists of ENHERTU, a HER2 directed ADC, and DATROWAY, a TROP2 directed ADC, which are being jointly developed and commercialized globally with AstraZeneca. Patritumab deruxtecan (HER3-DXd), a HER3 directed ADC, ifinatamab deruxtecan (I-DXd), a B7-H3 directed ADC, and raludotatug deruxtecan (R-DXd), a CDH6 directed ADC, are being jointly developed and commercialized globally with Merck & Co., Inc, Rahway, NJ, USA. DS-3939, a TA-MUC1 directed ADC, is being developed by Daiichi Sankyo.
The second Daiichi Sankyo ADC platform consists of a monoclonal antibody attached to a modified pyrrolobenzodiazepine (PBD) payload. DS-9606, a CLDN6 directed PBD ADC, is the first of several planned ADCs in clinical development utilizing this platform.
Ifinatamab deruxtecan, patritumab deruxtecan, raludotatug deruxtecan, DS-3939 and DS-9606 are investigational medicines that have not been approved for any indication in any country. Safety and efficacy have not been established.
ENHERTU U.S. Important Safety Information
Indications
ENHERTU is a HER2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of adult patients with:
Unresectable or metastatic HER2-positive (IHC 3+ or ISH positive) breast cancer who have received a prior anti-HER2-based regimen either: In the metastatic setting, or In the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy
In the metastatic setting, or
In the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy
Unresectable or metastatic: Hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer, as determined by an FDA-approved test, that has progressed on one or more endocrine therapies in the metastatic setting HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy
Hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer, as determined by an FDA-approved test, that has progressed on one or more endocrine therapies in the metastatic setting
HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy
Unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy This indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Locally advanced or metastatic HER2-positive (IHC 3+ or IHC 2+/ISH positive) gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen
Unresectable or metastatic HER2-positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options This indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
WARNING: INTERSTITIAL LUNG DISEASE and EMBRYO-FETAL TOXICITY
Contraindications
None.
Warnings and Precautions
Interstitial Lung Disease / Pneumonitis
Severe, life-threatening, or fatal interstitial lung disease (ILD), including pneumonitis, can occur in patients treated with ENHERTU. A higher incidence of Grade 1 and 2 ILD/pneumonitis has been observed in patients with moderate renal impairment. Advise patients to immediately report cough, dyspnea, fever, and/or any new or worsening respiratory symptoms. Monitor patients for signs and symptoms of ILD. Promptly investigate evidence of ILD. Evaluate patients with suspected ILD by radiographic imaging. Consider consultation with a pulmonologist. For asymptomatic ILD/pneumonitis (Grade 1), interrupt ENHERTU until resolved to Grade 0, then if resolved in ≤28 days from date of onset, maintain dose. If resolved in >28 days from date of onset, reduce dose 1 level. Consider corticosteroid treatment as soon as ILD/pneumonitis is suspected (e.g., ≥0.5 mg/kg/day prednisolone or equivalent). For symptomatic ILD/pneumonitis (Grade 2 or greater), permanently discontinue ENHERTU. Promptly initiate systemic corticosteroid treatment as soon as ILD/pneumonitis is suspected (e.g., ≥1 mg/kg/day prednisolone or equivalent) and continue for at least 14 days followed by gradual taper for at least 4 weeks.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, ILD occurred in 12% of patients. Median time to first onset was 5.5 months (range: 0.9 to 31.5). Fatal outcomes due to ILD and/or pneumonitis occurred in 0.9% of patients treated with ENHERTU.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, ILD occurred in 10% of patients. Median time to first onset was 2.8 months (range: 1.2 to 21).
Neutropenia
Severe neutropenia, including febrile neutropenia, can occur in patients treated with ENHERTU. Monitor complete blood counts prior to initiation of ENHERTU and prior to each dose, and as clinically indicated. For Grade 3 neutropenia (Absolute Neutrophil Count [ANC] <1.0 to 0.5 x 109/L), interrupt ENHERTU until resolved to Grade 2 or less, then maintain dose. For Grade 4 neutropenia (ANC <0.5 x 109/L), interrupt ENHERTU until resolved to Grade 2 or less, then reduce dose by 1 level. For febrile neutropenia (ANC <1.0 x 109/L and temperature >38.3º C or a sustained temperature of ≥38º C for more than 1 hour), interrupt ENHERTU until resolved, then reduce dose by 1 level.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, a decrease in neutrophil count was reported in 65% of patients. Nineteen percent had Grade 3 or 4 decreased neutrophil count. Median time to first onset of decreased neutrophil count was 22 days (range: 2 to 939). Febrile neutropenia was reported in 1.2% of patients.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, a decrease in neutrophil count was reported in 72% of patients. Fifty-one percent had Grade 3 or 4 decreased neutrophil count. Median time to first onset of decreased neutrophil count was 16 days (range: 4 to 187). Febrile neutropenia was reported in 4.8% of patients.
Left Ventricular Dysfunction
Patients treated with ENHERTU may be at increased risk of developing left ventricular dysfunction. Left ventricular ejection fraction (LVEF) decrease has been observed with anti-HER2 therapies, including ENHERTU. Assess LVEF prior to initiation of ENHERTU and at regular intervals during treatment as clinically indicated. Manage LVEF decrease through treatment interruption. When LVEF is >45% and absolute decrease from baseline is 10-20%, continue treatment with ENHERTU. When LVEF is 40-45% and absolute decrease from baseline is 20%, interrupt ENHERTU and repeat LVEF assessment within 3 weeks. If LVEF of 20% is confirmed, permanently discontinue ENHERTU. Permanently discontinue ENHERTU in patients with symptomatic congestive heart failure. Treatment with ENHERTU has not been studied in patients with a history of clinically significant cardiac disease or LVEF <50% prior to initiation of treatment.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, LVEF decrease was reported in 4.6% of patients, of which 0.6% were Grade 3 or 4.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, no clinical adverse events of heart failure were reported; however, on echocardiography, 8% were found to have asymptomatic Grade 2 decrease in LVEF.
Embryo-Fetal Toxicity
ENHERTU can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risks to a fetus. Verify the pregnancy status of females of reproductive potential prior to the initiation of ENHERTU. Advise females of reproductive potential to use effective contraception during treatment and for 7 months after the last dose of ENHERTU. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose of ENHERTU.
Additional Dose Modifications
Thrombocytopenia
For Grade 3 thrombocytopenia (platelets <50 to 25 x 109/L) interrupt ENHERTU until resolved to Grade 1 or less, then maintain dose. For Grade 4 thrombocytopenia (platelets <25 x 109/L) interrupt ENHERTU until resolved to Grade 1 or less, then reduce dose by 1 level.
Adverse Reactions
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
The pooled safety population reflects exposure to ENHERTU 5.4 mg/kg intravenously every 3 weeks in 2233 patients in Study DS8201-A-J101 (NCT02564900), DESTINY-Breast01, DESTINY-Breast02, DESTINYBreast03, DESTINY-Breast04, DESTINY-Breast06, DESTINY-Lung01, DESTINY-Lung02, DESTINY-CRC02, and DESTINY-PanTumor02. Among these patients, 67% were exposed for >6 months and 38% were exposed for >1 year. In this pooled safety population, the most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count (73%), nausea (72%), decreased hemoglobin (67%), decreased neutrophil count (65%), decreased lymphocyte count (60%), fatigue (55%), decreased platelet count (48%), increased aspartate aminotransferase (46%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (39%), vomiting (38%), alopecia (37%), constipation (32%), decreased blood potassium (32%), decreased appetite (31%), diarrhea (30%), and musculoskeletal pain (24%).
HER2-Positive Metastatic Breast Cancer
DESTINY-Breast03
The safety of ENHERTU was evaluated in 257 patients with unresectable or metastatic HER2-positive breast cancer who received at least 1 dose of ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast03. The median duration of treatment was 14 months (range: 0.7 to 30) for patients who received ENHERTU.
Serious adverse reactions occurred in 19% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were vomiting, ILD, pneumonia, pyrexia, and urinary tract infection. Fatalities due to adverse reactions occurred in 0.8% of patients including COVID-19 and sudden death (1 patient each).
ENHERTU was permanently discontinued in 14% of patients, of which ILD/pneumonitis accounted for 8%. Dose interruptions due to adverse reactions occurred in 44% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, leukopenia, anemia, thrombocytopenia, pneumonia, nausea, fatigue, and ILD/pneumonitis. Dose reductions occurred in 21% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were nausea, neutropenia, and fatigue.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea (76%), decreased white blood cell count (74%), decreased neutrophil count (70%), increased aspartate aminotransferase (67%), decreased hemoglobin (64%), decreased lymphocyte count (55%), increased alanine aminotransferase (53%), decreased platelet count (52%), fatigue (49%), vomiting (49%), increased blood alkaline phosphatase (49%), alopecia (37%), decreased blood potassium (35%), constipation (34%), musculoskeletal pain (31%), diarrhea (29%), decreased appetite (29%), headache (22%), respiratory infection (22%), abdominal pain (21%), increased blood bilirubin (20%), and stomatitis (20%).
HER2-Low and HER2-Ultralow Metastatic Breast Cancer
DESTINY-Breast06
The safety of ENHERTU was evaluated in 434 patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast06. The median duration of treatment was 11 months (range: 0.4 to 39.6) for patients who received ENHERTU.
Serious adverse reactions occurred in 20% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, COVID-19, febrile neutropenia, and hypokalemia. Fatalities due to adverse reactions occurred in 2.8% of patients including ILD (0.7%); sepsis (0.5%); and COVID-19 pneumonia, bacterial meningoencephalitis, neutropenic sepsis, peritonitis, cerebrovascular accident, general physical health deterioration (0.2% each).
ENHERTU was permanently discontinued in 14% of patients. The most frequent adverse reaction (>2%) associated with permanent discontinuation was ILD/pneumonitis. Dose interruptions due to adverse reactions occurred in 48% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were COVID-19, decreased neutrophil count, anemia, pyrexia, pneumonia, decreased white blood cell count, and ILD. Dose reductions occurred in 25% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were nausea, fatigue, decreased platelet count, and decreased neutrophil count.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count (86%), decreased neutrophil count (75%), nausea (70%), decreased hemoglobin (69%), decreased lymphocyte count (66%), fatigue (53%), decreased platelet count (48%), alopecia (48%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (43%), increased aspartate aminotransferase (41%), decreased blood potassium (35%), diarrhea (34%), vomiting (34%), constipation (32%), decreased appetite (26%), COVID-19 (26%), and musculoskeletal pain (24%).
DESTINY-Breast04
The safety of ENHERTU was evaluated in 371 patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast04. The median duration of treatment was 8 months (range: 0.2 to 33) for patients who received ENHERTU.
Serious adverse reactions occurred in 28% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, pneumonia, dyspnea, musculoskeletal pain, sepsis, anemia, febrile neutropenia, hypercalcemia, nausea, pyrexia, and vomiting. Fatalities due to adverse reactions occurred in 4% of patients including ILD/pneumonitis (3 patients); sepsis (2 patients); and ischemic colitis, disseminated intravascular coagulation, dyspnea, febrile neutropenia, general physical health deterioration, pleural effusion, and respiratory failure (1 patient each).
ENHERTU was permanently discontinued in 16% of patients, of which ILD/pneumonitis accounted for 8%. Dose interruptions due to adverse reactions occurred in 39% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, fatigue, anemia, leukopenia, COVID-19, ILD/pneumonitis, increased transaminases, and hyperbilirubinemia. Dose reductions occurred in 23% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, thrombocytopenia, and neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea (76%), decreased white blood cell count (70%), decreased hemoglobin (64%), decreased neutrophil count (64%), decreased lymphocyte count (55%), fatigue (54%), decreased platelet count (44%), alopecia (40%), vomiting (40%), increased aspartate aminotransferase (38%), increased alanine aminotransferase (36%), constipation (34%), increased blood alkaline phosphatase (34%), decreased appetite (32%), musculoskeletal pain (32%), diarrhea (27%), and decreased blood potassium (25%).
HER2-Mutant Unresectable or Metastatic NSCLC (5.4 mg/kg)
DESTINY-Lung02 evaluated 2 dose levels (5.4 mg/kg [n=101] and 6.4 mg/kg [n=50]); however, only the results for the recommended dose of 5.4 mg/kg intravenously every 3 weeks are described below due to increased toxicity observed with the higher dose in patients with NSCLC, including ILD/pneumonitis.
The safety of ENHERTU was evaluated in 101 patients with HER2-mutant unresectable or metastatic NSCLC who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks until disease progression or unacceptable toxicity in DESTINY-Lung02. Nineteen percent of patients were exposed for >6 months.
Serious adverse reactions occurred in 30% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, thrombocytopenia, dyspnea, nausea, pleural effusion, and increased troponin I. Fatality occurred in 1 patient with suspected ILD/pneumonitis (1%).
ENHERTU was permanently discontinued in 8% of patients. Adverse reactions which resulted in permanent discontinuation of ENHERTU were ILD/pneumonitis, diarrhea, decreased blood potassium, hypomagnesemia, myocarditis, and vomiting. Dose interruptions of ENHERTU due to adverse reactions occurred in 23% of patients. Adverse reactions which required dose interruption (>2%) included neutropenia and ILD/pneumonitis. Dose reductions due to an adverse reaction occurred in 11% of patients.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea (61%), decreased white blood cell count (60%), decreased hemoglobin (58%), decreased neutrophil count (52%), decreased lymphocyte count (43%), decreased platelet count (40%), decreased albumin (39%), increased aspartate aminotransferase (35%), increased alanine aminotransferase (34%), fatigue (32%), constipation (31%), decreased appetite (30%), vomiting (26%), increased alkaline phosphatase (22%), and alopecia (21%).
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
The safety of ENHERTU was evaluated in 187 patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma in DESTINY-Gastric01. Patients intravenously received at least 1 dose of either ENHERTU (N=125) 6.4 mg/kg every 3 weeks or either irinotecan (N=55) 150 mg/m2 biweekly or paclitaxel (N=7) 80 mg/m2 weekly for 3 weeks. The median duration of treatment was 4.6 months (range: 0.7 to 22.3) for patients who received ENHERTU.
Serious adverse reactions occurred in 44% of patients receiving ENHERTU 6.4 mg/kg. Serious adverse reactions in >2% of patients who received ENHERTU were decreased appetite, ILD, anemia, dehydration, pneumonia, cholestatic jaundice, pyrexia, and tumor hemorrhage. Fatalities due to adverse reactions occurred in 2.4% of patients: disseminated intravascular coagulation, large intestine perforation, and pneumonia occurred in 1 patient each (0.8%).
ENHERTU was permanently discontinued in 15% of patients, of which ILD accounted for 6%. Dose interruptions due to adverse reactions occurred in 62% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, anemia, decreased appetite, leukopenia, fatigue, thrombocytopenia, ILD, pneumonia, lymphopenia, upper respiratory tract infection, diarrhea, and decreased blood potassium. Dose reductions occurred in 32% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were neutropenia, decreased appetite, fatigue, nausea, and febrile neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased hemoglobin (75%), decreased white blood cell count (74%), decreased neutrophil count (72%), decreased lymphocyte count (70%), decreased platelet count (68%), nausea (63%), decreased appetite (60%), increased aspartate aminotransferase (58%), fatigue (55%), increased blood alkaline phosphatase (54%), increased alanine aminotransferase (47%), diarrhea (32%), decreased blood potassium (30%), vomiting (26%), constipation (24%), increased blood bilirubin (24%), pyrexia (24%), and alopecia (22%).
HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
The safety of ENHERTU was evaluated in 347 adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors who received ENHERTU 5.4 mg/kg intravenously once every 3 weeks in DESTINY-Breast01, DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02. The median duration of treatment was 8.3 months (range 0.7 to 30.2).
Serious adverse reactions occurred in 34% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were sepsis, pneumonia, vomiting, urinary tract infection, abdominal pain, nausea, pneumonitis, pleural effusion, hemorrhage, COVID-19, fatigue, acute kidney injury, anemia, cellulitis, and dyspnea. Fatalities due to adverse reactions occurred in 6.3% of patients including ILD/pneumonitis (2.3%), cardiac arrest (0.6%), COVID-19 (0.6%), and sepsis (0.6%). The following events occurred in 1 patient each (0.3%): acute kidney injury, cerebrovascular accident, general physical health deterioration, pneumonia, and hemorrhagic shock.
ENHERTU was permanently discontinued in 15% of patients, of which ILD/pneumonitis accounted for 10%. Dose interruptions due to adverse reactions occurred in 48% of patients. The most frequent adverse reactions (>2%) associated with dose interruption were decreased neutrophil count, anemia, COVID-19, fatigue, decreased white blood cell count, and ILD/pneumonitis. Dose reductions occurred in 27% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, decreased neutrophil count, ILD/pneumonitis, and diarrhea.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count (75%), nausea (69%), decreased hemoglobin (67%), decreased neutrophil count (66%), fatigue (59%), decreased lymphocyte count (58%), decreased platelet count (51%), increased aspartate aminotransferase (45%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (36%), vomiting (35%), decreased appetite (34%), alopecia (34%), diarrhea (31%), decreased blood potassium (29%), constipation (28%), decreased sodium (22%), stomatitis (20%), and upper respiratory tract infection (20%).
Use in Specific Populations
Pregnancy: ENHERTU can cause fetal harm when administered to a pregnant woman. Advise patients of the potential risks to a fetus. There are clinical considerations if ENHERTU is used in pregnant women, or if a patient becomes pregnant within 7 months after the last dose of ENHERTU.
Lactation: There are no data regarding the presence of ENHERTU in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with ENHERTU and for 7 months after the last dose.
Females and Males of Reproductive Potential: Pregnancy testing : Verify pregnancy status of females of reproductive potential prior to initiation of ENHERTU. Contraception : Females : ENHERTU can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with ENHERTU and for 7 months after the last dose. Males : Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose. Infertility : ENHERTU may impair male reproductive function and fertility.
Pediatric Use: Safety and effectiveness of ENHERTU have not been established in pediatric patients.
Geriatric Use: Of the 1741 patients with HER2-positive, HER2-low, or HER2-ultralow breast cancer treated with ENHERTU 5.4 mg/kg, 24% were ≥65 years and 4.9% were ≥75 years. No overall differences in efficacy within clinical studies were observed between patients ≥65 years of age compared to younger patients. There was a higher incidence of Grade 3-4 adverse reactions observed in patients aged ≥65 years (61%) as compared to younger patients (52%). Of the 101 patients with HER2-mutant unresectable or metastatic NSCLC treated with ENHERTU 5.4 mg/kg, 40% were ≥65 years and 8% were ≥75 years. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients. Of the 125 patients with HER2-positive locally advanced or metastatic gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg in DESTINY-Gastric01, 56% were ≥65 years and 14% were ≥75 years. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients. Of the 192 patients with HER2-positive (IHC 3+) unresectable or metastatic solid tumors treated with ENHERTU 5.4 mg/kg in DESTINY-PanTumor02, DESTINY-Lung01, or DESTINY-CRC02, 39% were ≥65 years and 9% were ≥75 years. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients.
Renal Impairment: A higher incidence of Grade 1 and 2 ILD/pneumonitis has been observed in patients with moderate renal impairment. Monitor patients with moderate renal impairment more frequently. The recommended dosage of ENHERTU has not been established for patients with severe renal impairment (CLcr <30 mL/min).
Hepatic Impairment: In patients with moderate hepatic impairment, due to potentially increased exposure, closely monitor for increased toxicities related to the topoisomerase inhibitor, DXd. The recommended dosage of ENHERTU has not been established for patients with severe hepatic impairment (total bilirubin >3 times ULN and any AST).
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or fda.gov/medwatch.
Please see accompanying full Prescribing Information, including Boxed WARNINGS, and Medication Guide.
About Daiichi Sankyo
Daiichi Sankyo is an innovative global healthcare company contributing to the sustainable development of society that discovers, develops and delivers new standards of care to enrich the quality of life around the world. With more than 120 years of experience, Daiichi Sankyo leverages its world-class science and technology to create new modalities and innovative medicines for people with cancer, cardiovascular and other diseases with high unmet medical need. For more information, please visit www.daiichisankyo.com.
References:
1 World Health Organization. Breast Fact Sheet. Accessed October 2025.
2 Cheng X, et al. Genes. (Basel). 2024;15(7):903.
3 Tarantino P, et al. J An Onc. 2023;34(8):645-659.
4 Mahtani R, et al. Cancers (Basel). 2025;17(119):1848.
5 Spring LM, et al. Clin Cancer Res. 2020;26(12):2838-2284.
6 Schneeweiss A, et al. Annals of Oncol. 2013; 24:2278-2284.
7 Swain S, et al. Annals of Oncol. 2018; 29:646-653.
8 Huober J, et al. J Clin Oncol. 2022; 40:2946-2956.
9 Masuda N, et al. Breast Cancer Res Treat. 2020; 180:135-146.
10 Gao H, et al. Presented ASCO Annual Meeting 2025.
11 Ban M, et al. Breast Care. 2020; 15:560-569.
12 Vuger A, et al. Breast. 2022:65:67-76.
13 Cai F, et al. Mol Clin Oncol. 2019;11(1):15-23.
Clinical ResultPhase 3Drug ApprovalLicense out/inADC
17 Oct 2025
A confirmed objective response rate of 68.2% and a disease control rate of 95.5% was observed with Daiichi Sankyo and AstraZeneca’s DATROWAY in combination with rilvegostomig in previously untreated, cisplatin ineligible patients
TROPION-Urothelial03 phase 2/3 trial initiated to further evaluate the role of DATROWAY plus platinum-based chemotherapy in previously treated metastatic urothelial cancer
TOKYO & BASKING RIDGE, N.J.--(BUSINESS WIRE)--Initial results from one sub-study of the TROPION-PanTumor03 phase 2 trial showed that DATROWAY® (datopotamab deruxtecan) plus rilvegostomig showed promising tumor responses and disease control as first-line and second-line combination therapy in patients with locally advanced or metastatic urothelial cancer. These results were presented today as a mini oral session (3072MO) at the 2025 European Society for Medical Oncology (#ESMO25) Congress.
DATROWAY is a specifically engineered TROP2 directed DXd antibody drug conjugate (ADC) discovered by Daiichi Sankyo (TSE: 4568) and being jointly developed and commercialized by Daiichi Sankyo and AstraZeneca (LSE/STO/Nasdaq: AZN). Rilvegostomig is AstraZeneca’s PD-1/TIGIT bispecific antibody.
In a sub-study of patients with locally advanced or metastatic urothelial cancer, DATROWAY plus rilvegostomig was evaluated in both the first-line and second-line treatment settings. As first-line treatment for patients not eligible for cisplatin (n=22), DATROWAY plus rilvegostomig demonstrated a confirmed objective response rate (ORR) of 68.2% (95% confidence interval [CI]: 45.1-86.1) and a disease control rate (DCR) of 95.5% (80% CI: 83.4-99.5). Median progression free survival (PFS) was not reached in the first-line setting and the PFS rate was 73.5% (95% CI: 46.5-88.4) at 12 months. As second-line treatment for patients who previously received platinum-based chemotherapy and no prior treatment with immunotherapy (n=18), DATROWAY plus rilvegostomig demonstrated a confirmed ORR of 38.9% (95% CI: 17.3-64.3) and a DCR of 83.3% (80% CI: 66.6-93.7). Median PFS was 12.5 months (95% CI: 4.2-NR) and the PFS rate was 60.0% (95% CI: 33.7–78.7) at 12 months. Median duration of response (DoR) was not yet reached in either setting.
“Despite recent advances in the treatment of metastatic urothelial cancer, there are limited treatment options in both the first-line and second-line settings and many patients still experience disease progression after first-line therapy,” said Sun Young Rha, MD, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Republic of Korea. “The response rates seen with TROPION-PanTumor03 across the first-line and second-line settings, including the disease control rate of 95.5% observed in the first-line setting, highlight the potential of datopotamab deruxtecan plus rilvegostomig and warrant further evaluation across lines of therapy.”
The safety profile of DATROWAY plus rilvegostomig was consistent with the known safety profiles of each agent. Grade 3 or higher treatment-related adverse events (TRAEs) were observed in four of 22 patients (18.2%) receiving first-line treatment and in seven of 18 patients (38.9%) receiving second-line treatment. The most common grade 3 or higher TRAEs occurring in patients treated with DATROWAY plus rilvegostomig in the first-line (n=22) and second-line (n=18) settings, respectively, were increased amylase (13.6%, 16.7%), neutropenia (4.5%, 5.6%), stomatitis (0%, 5.6%), decreased appetite (0%, 5.6%) and anemia (0%, 5.6%). There was one (4.5%) interstitial lung disease (ILD) event adjudicated as drug-related in first-line and two (11.1%) in second-line setting with no grade 3 or higher ILD events observed.
Daiichi Sankyo has initiated the TROPION-Urothelial03 phase 2/3 trial evaluating DATROWAY plus platinum-based chemotherapy compared to gemcitabine and platinum-based chemotherapy in patients with metastatic urothelial carcinoma following progression during or after treatment with enfortumab vedotin in combination with pembrolizumab.
“The five-year survival rate for patients with metastatic urothelial cancer remains very low, underscoring the urgent need for new therapeutic options in this difficult-to-treat population,” said Abderrahmane Laadem, MD, Head, Late-Stage Oncology Development, Daiichi Sankyo. “These results from TROPION-PanTumor03, coupled with results from TROPION-PanTumor01, support further clinical development of DATROWAY in urothelial cancer and we have initiated the TROPION-Urothelial03 phase 2/3 trial, our first pivotal trial outside of breast and lung cancer.”
“These initial TROPION-PanTumor03 results offer further support for our strategy of combining the potency of DATROWAY with the dual PD-1 and TIGIT blockade of rilvegostomig to enhance patients’ immune responses and ultimately, improve outcomes,” said Leora Horn, MD, MSC, FRCPC, Senior Vice President, Late Development Oncology, AstraZeneca. “We are encouraged by the responses observed in patients with metastatic urothelial cancer and hope to further evaluate this combination in the early-line setting.”
In TROPION-PanTumor03, patients were enrolled across seven sub-studies that evaluated DATROWAY as monotherapy or in combination with other approved or investigational cancer medicines. In a sub-study evaluating patients with locally advanced or metastatic urothelial cancer (sub-study 6, cohort 6B [n=40]), patients received DATROWAY in combination with rilvegostomig in either the first-line setting (cisplatin ineligible patients with no prior systemic therapy, or progression more than 12 months after neoadjuvant or adjuvant therapy) or the second-line setting (previous platinum-based chemotherapy in the locally advanced or metastatic setting, or progression less than 12 months after platinum-based neoadjuvant or adjuvant therapy). Of the second-line patients who were not previously treated with immunotherapy (n=18), six had previous treatment in the adjuvant or neoadjuvant setting with progression within 12 months and 12 had been treated in the metastatic setting. Median duration of follow-up was 10.8 months and 9.7 months in the first-line and second-line setting, respectively.
Summary of Sub-study 6, Cohort 6B of TROPION-PanTumor03 Efficacy Results
Efficacy Measure
First-Line Subset (n=22)
Second-Line Subset (n=18)
Confirmed ORR,i,ii (%) (95% CI)
68.2% (45.1–86.1)
38.9% (17.3–64.3)
CR, n (%)
1 (4.5%)
0
PR, n (%)
14 (63.6%)
7 (38.9%)
SD, n (%)
6 (27.3%)
8 (44.4%)
PD, n (%)
1 (4.6%)iii
3 (16%)
DCR at 12 weeks,iv (%) (80% CI)
95.5% (83.4–99.5)
83.3% (66.6–93.7)
DoR, median ii (months) (95% CI)
NR (9.4 months–NR)
NR (6.9 months–NR)
PFS medianii (months) (95% CI)
NR (10.8 months–NR)
12.5 months (4.2 months–NR)
CI, confidence interval; CR, complete response; DCR, disease control rate; DoR, duration of response; NR, not reached; ORR, objective response rate; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease
iORR is CR+ PR
iiAs assessed by investigator
iiiPatient has no post-baseline assessment and died less than 13 weeks after first dose
ivProportion of patients who had achieved a CR or PR in the first 13 weeks or who had SD for at least 11 weeks after start of treatment as assessed by investigator
About TROPION-PanTumor03
TROPION-PanTumor03 is a global, multicenter, multi-cohort, open-label, phase 2 trial evaluating the efficacy and safety of DATROWAY as monotherapy and in combination with various approved or investigational cancer medicines across seven sub-studies in several metastatic solid cancers, including endometrial, gastric, prostate, ovarian, colorectal, urothelial and biliary tract cancer.
Cohort 6B is evaluating DATROWAY (6 mg/kg) plus rilvegostomig as first-line and second-line combination therapies in patients with locally advanced or metastatic urothelial cancer. The primary endpoint is ORR as assessed by investigator as well as safety and tolerability. Secondary endpoints include investigator-assessed DCR, DoR, PFS as assessed by investigator as well as pharmacokinetics.
TROPION-PanTumor03 will enroll approximately 580 patients across all sub-studies in Asia, Europe and North America. For more information visit ClinicalTrials.gov.
Rilvegostomig is AstraZeneca’s PD-1/TIGIT bispecific antibody. The TIGIT component of rilvegostomig is derived from the clinical-stage anti-TIGIT antibody, COM902, developed by Compugen Ltd. (Nasdaq/TASE: CGEN).
About TROPION-Urothelial03
TROPION-Urothelial03 is a global, multicenter, randomized, open-label phase 2/3 trial evaluating the efficacy and safety of DATROWAY plus platinum-based chemotherapy (carboplatin or cisplatin) versus gemcitabine plus platinum-based chemotherapy in patients with locally advanced or metastatic urothelial carcinoma with disease progression during or after enfortumab vedotin plus pembrolizumab combination treatment. The phase 2 part of the trial will determine the recommended dose of DATROWAY (4 mg/kg or 6 mg/kg) in combination with carboplatin or cisplatin. The phase 3 part will evaluate the efficacy and safety of DATROWAY at the recommended dose plus carboplatin or cisplatin versus gemcitabine plus carboplatin or cisplatin.
The primary endpoint of the phase 2 part of TROPION-Urothelial03 is ORR assessed by investigator. Secondary endpoints include DoR, PFS, DCR, time to response (TTR), overall survival (OS) and safety.
The dual primary endpoints of the phase 3 part of TROPION-Urothelial03 are PFS assessed by blinded independent central review (BICR) and OS. Secondary endpoints include PFS by investigator, ORR, DoR, TTR, DCR, each assessed by BICR and by investigator, and safety.
TROPION-Urothelial03 will enroll approximately 630 patients at sites in Asia, Europe, North America, Oceania and South America. For more information visit ClinicalTrials.gov.
About Urothelial (Bladder) Cancer
More than 610,000 bladder cancer cases were diagnosed globally in 2022.1 The most common type of bladder cancer is urothelial cancer, accounting for approximately 90% of cases.2 While the five-year survival rate for localized bladder cancer is more than 70%, survival decreases to approximately 9% in the metastatic setting.3,4
While targeted therapies have improved outcomes in the first-line metastatic setting, disease progression is common and treatment options in the second-line setting are limited.5
TROP2 is a protein broadly expressed in several solid tumors including urothelial cancer.6 TROP2 expression is positively correlated with disease severity in patients with urothelial cancer.7
About DATROWAY
DATROWAY (datopotamab deruxtecan; datopotamab deruxtecan-dlnk in the U.S. only) is a TROP2 directed ADC. Designed using Daiichi Sankyo’s proprietary DXd ADC Technology, DATROWAY is one of six DXd ADCs in the oncology pipeline of Daiichi Sankyo, and one of the most advanced programs in AstraZeneca’s ADC scientific platform. DATROWAY is comprised of a humanized anti-TROP2 IgG1 monoclonal antibody, developed in collaboration with Sapporo Medical University, attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers.
DATROWAY is approved in more than 35 countries/regions for the treatment of adult patients with unresectable or metastatic HR positive, HER2 negative (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer who have received prior endocrine-based therapy and chemotherapy for unresectable or metastatic disease based on the results from the TROPION-Breast01 trial.
DATROWAY (6 mg/kg) is approved in Russia and the U.S. for the treatment of adult patients with locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC) who have received prior EGFR-directed therapy and platinum-based chemotherapy based on the results from the TROPION-Lung05 and TROPION-Lung01 trials. Continued approval for this indication in the U.S. may be contingent upon verification and description of clinical benefit in the confirmatory trial.
About the DATROWAY Clinical Development Program
A comprehensive global clinical development program is underway with more than 20 trials evaluating the efficacy and safety of DATROWAY across multiple cancers, including NSCLC, triple negative breast cancer and urothelial cancer. The program includes eight phase 3 trials in lung cancer and five phase 3 trials in breast cancer evaluating DATROWAY as a monotherapy and in combination with other cancer treatments in various settings.
About the Daiichi Sankyo and AstraZeneca Collaboration
Daiichi Sankyo and AstraZeneca entered into a global collaboration to jointly develop and commercialize ENHERTU® in March 2019 and DATROWAY in July 2020, except in Japan where Daiichi Sankyo maintains exclusive rights for each ADC. Daiichi Sankyo is responsible for the manufacturing and supply of ENHERTU and DATROWAY.
About the ADC Portfolio of Daiichi Sankyo
The Daiichi Sankyo ADC portfolio consists of seven ADCs in clinical development crafted from two distinct ADC technology platforms discovered in-house by Daiichi Sankyo.
The ADC platform furthest in clinical development is Daiichi Sankyo’s DXd ADC Technology where each ADC consists of a monoclonal antibody attached to a number of topoisomerase I inhibitor payloads (an exatecan derivative, DXd) via tetrapeptide-based cleavable linkers. The DXd ADC portfolio currently consists of ENHERTU, a HER2 directed ADC, and DATROWAY, a TROP2 directed ADC, which are being jointly developed and commercialized globally with AstraZeneca. Patritumab deruxtecan (HER3-DXd), a HER3 directed ADC, ifinatamab deruxtecan (I-DXd), a B7-H3 directed ADC, and raludotatug deruxtecan (R-DXd), a CDH6 directed ADC, are being jointly developed and commercialized globally with Merck & Co., Inc, Rahway, NJ, USA. DS-3939, a TA-MUC1 directed ADC, is being developed by Daiichi Sankyo.
The second Daiichi Sankyo ADC platform consists of a monoclonal antibody attached to a modified pyrrolobenzodiazepine (PBD) payload. DS-9606, a CLDN6 directed PBD ADC, is the first of several planned ADCs in clinical development utilizing this platform.
Ifinatamab deruxtecan, patritumab deruxtecan, raludotatug deruxtecan, DS-3939 and DS-9606 are investigational medicines that have not been approved for any indication in any country. Safety and efficacy have not been established.
DATROWAY U.S. Important Safety Information
Indications
DATROWAY® is a Trop-2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of:
adult patients with locally advanced or metastatic epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) who have received prior EGFR-directed therapy and platinum-based chemotherapy. This indication is approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trial.
adult patients with unresectable or metastatic, hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative (IHC 0, IHC 1+ or IHC 2+/ISH-) breast cancer who have received prior endocrine-based therapy and chemotherapy for unresectable or metastatic disease.
Contraindications
None.
Warnings and Precautions
Interstitial Lung Disease/Pneumonitis
DATROWAY can cause severe, life-threatening, or fatal interstitial lung disease (ILD) or pneumonitis.
Locally Advanced or Metastatic NSCLC
In the pooled safety population of 484 patients with NSCLC from TROPION-Lung01, TROPION-Lung05, and TROPION-PanTumor01, ILD/pneumonitis occurred in 7% of patients treated with DATROWAY, including 0.6% of patients with Grade 3 and 0.4% with Grade 4. There were 8 (1.7%) fatal cases. The median time to onset for ILD was 1.4 months (range: 0.2 months to 9 months). Eleven patients (2.3%) had DATROWAY withheld and 20 patients (4.1%) permanently discontinued DATROWAY due to ILD/pneumonitis. Systemic corticosteroids were required in 79% (26/33) of patients with ILD/pneumonitis. ILD/pneumonitis resolved in 45% of patients.
Unresectable or Metastatic Breast Cancer
In the pooled safety population of 443 patients with breast cancer from TROPION-Breast01 and TROPION-PanTumor01, ILD/pneumonitis occurred in 3.6% of patients treated with DATROWAY, including 0.7% of patients with Grade 3. There was one fatal case (0.2%). The median time to onset for ILD was 2.8 months (range: 1.1 months to 10.8 months). Four patients (0.9%) had DATROWAY withheld and 7 patients (1.6%) permanently discontinued DATROWAY due to ILD/pneumonitis. Systemic corticosteroids were required in 60% (9/15) of patients with ILD/pneumonitis. ILD/pneumonitis resolved in 40% of patients.
Patients were excluded from clinical studies for a history of ILD/pneumonitis requiring treatment with steroids or for ongoing ILD/pneumonitis.
Monitor patients for new or worsening respiratory symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever) during treatment with DATROWAY. For asymptomatic (Grade 1) ILD/pneumonitis, consider corticosteroid treatment (e.g., ≥0.5 mg/kg/day prednisolone or equivalent). For symptomatic ILD/pneumonitis (Grade 2 or greater), promptly initiate systemic corticosteroid treatment (e.g., ≥1 mg/kg/day prednisolone or equivalent) and continue for at least 14 days followed by gradual taper for at least 4 weeks.
Withhold DATROWAY in patients with suspected ILD/pneumonitis and permanently discontinue DATROWAY if ≥Grade 2 ILD/pneumonitis is confirmed.
Ocular Adverse Reactions
DATROWAY can cause ocular adverse reactions including dry eye, keratitis, blepharitis, meibomian gland dysfunction, increased lacrimation, conjunctivitis, and blurred vision.
In the pooled safety population, ocular adverse reactions occurred in 36% of patients treated with DATROWAY. Twenty patients (2.2%) experienced Grade 3 ocular adverse reactions, which included keratitis, dry eye, and blurred vision, and one patient experienced a Grade 4 ocular adverse reaction of conjunctival hemorrhage. The most common (≥5%) ocular adverse reactions were dry eye (17%), keratitis (14%), and increased lacrimation (7%). The median time to onset for ocular adverse reactions was 2.3 months (range: 0.03 months to 23.2 months). Of the patients who experienced ocular adverse reactions, 39% had complete resolution, and 10% had partial improvement (defined as a decrease in severity by one or more grades from the worst grade at last follow up). Ocular adverse reactions led to dosage interruption in 3.6% of patients, dosage reductions in 2.5% of patients, and permanent discontinuation of DATROWAY in 1% of patients.
Patients with clinically significant corneal disease were excluded from clinical studies.
Advise patients to use preservative-free lubricant eye drops several times daily for prophylaxis. Advise patients to avoid use of contact lenses unless directed by an eye care professional.
Refer patients to an eye care professional for an ophthalmic exam including visual acuity testing, slit lamp examination (with fluorescein staining), intraocular pressure, and fundoscopy at treatment initiation, annually while on treatment, at end of treatment, and as clinically indicated.
Promptly refer patients to an eye care professional for any new or worsening ocular adverse reactions. Monitor patients for ocular adverse reactions during treatment with DATROWAY, and if diagnosis is confirmed, withhold, reduce the dose, or permanently discontinue DATROWAY based on severity.
Stomatitis
DATROWAY can cause stomatitis, including mouth ulcers and oral mucositis.
In the pooled safety population, stomatitis occurred in 63% of patients treated with DATROWAY, including 8% of patients with Grade 3 events and one patient with a Grade 4 reaction. The median time to first onset of stomatitis was 0.5 months (range: 0.03 months to 18.6 months). Stomatitis led to dosage interruption in 6% of patients, dosage reductions in 11% of patients, and permanent discontinuation of DATROWAY in 0.5% of patients.
In patients who received DATROWAY in TROPION-Breast01, 39% used a mouthwash containing corticosteroid for management or prophylaxis of stomatitis/oral mucositis at any time during the treatment.
Advise patients to use a steroid-containing mouthwash for prophylaxis and treatment of stomatitis. Instruct the patient to hold ice chips or ice water in the mouth throughout the infusion of DATROWAY.
Monitor patients for signs and symptoms of stomatitis. If stomatitis occurs, increase the frequency of mouthwash and administer other topical treatments as clinically indicated. Based on the severity of the adverse reaction, withhold, reduce the dose, or permanently discontinue DATROWAY.
Embryo-Fetal Toxicity
Based on its mechanism of action, DATROWAY can cause embryo-fetal harm when administered to a pregnant woman because the topoisomerase inhibitor component of DATROWAY, DXd, is genotoxic and affects actively dividing cells.
Advise patients of the potential risk to a fetus. Advise female patients of reproductive potential to use effective contraception during treatment with DATROWAY and for 7 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with DATROWAY and for 4 months after the last dose.
Adverse Reactions
The pooled safety population described in WARNINGS AND PRECAUTIONS reflects exposure to DATROWAY in 927 patients as a single agent at 6 mg/kg administered as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity. This included 137 patients with NSCLC in TROPION-Lung05, 297 patients with NSCLC in TROPION-Lung01, 360 patients with HR-positive, HER2-negative breast cancer in TROPION-Breast01, and 50 patients with NSCLC and 83 patients with breast cancer in TROPION-PanTumor01 (NCT03401385). Among 927 patients who received DATROWAY, 45% were exposed for 6 months or longer and 19% were exposed for greater than one year. In this pooled safety population, the most common (≥20%) adverse reactions were stomatitis (63%), nausea (52%), fatigue (45%), alopecia (38%), constipation (28%), decreased appetite (23%), rash (23%), vomiting (22%), and musculoskeletal pain (20%). In this pooled safety population, the most common (≥2%) Grade 3 or 4 laboratory abnormalities were decreased lymphocytes (9%) and decreased hemoglobin (3.5%).
Locally Advanced or Metastatic EGFR-Mutated Non-Small Cell Lung Cancer
TROPION-Lung05, TROPION-Lung01, TROPION-PanTumor01
The safety of DATROWAY was evaluated in 125 patients with EGFR-mutated NSCLC who received DATROWAY 6 mg/kg administered as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity in TROPION-Lung05 and TROPION-Lung01 as well as TROPION-PanTumor01 (NCT03401385). Among these patients, the median duration of treatment was 6.1 months (range 0.7 months to 41.7 months).
The median age was 63 years (range: 36 to 81), 56% of patients were <65 years, 62% of patients were female; 66% were Asian, 26% were White, 0.8% were Black, 6% were other races; and 2.4% were of Hispanic ethnicity.
Serious adverse reactions occurred in 26% of patients who received DATROWAY. Serious adverse reactions in >1% of patients who received DATROWAY were COVID-19 (4%), stomatitis (2.4%), and pneumonia (1.6%). Fatal adverse reactions occurred in 1.6% of patients who received DATROWAY, due to death not otherwise specified.
Permanent discontinuation of DATROWAY due to an adverse reaction occurred in 8% of patients. Adverse reactions which resulted in permanent discontinuation of DATROWAY in >1% of patients included ILD/pneumonitis (2.4%) and abnormal hepatic function (1.6%).
Dosage interruptions of DATROWAY due to an adverse reaction occurred in 43% of patients. Adverse reactions which required dosage interruption in >1% of patients included COVID-19 (13%), stomatitis (7%), fatigue (6%), pneumonia (4%), anemia (2.4%), amylase increased (2.4%), keratitis (2.4%), ILD/pneumonitis (1.6%), decreased appetite (1.6%), dyspnea (1.6%), rash (1.6%), and infusion-related reaction (1.6%).
Dose reductions of DATROWAY due to an adverse reaction occurred in 26% of patients. Adverse reactions which required dose reduction in >1% of patients included stomatitis (14%), keratitis (1.6%), fatigue (1.6%), decreased weight (1.6%) and COVID-19 (1.6%).
The most common (≥20%) adverse reactions, including laboratory abnormalities, were stomatitis (71%), nausea (50%), alopecia (49%), fatigue (42%), decreased hemoglobin (34%), decreased lymphocytes (32%), constipation (31%), increased calcium (31%), increased AST (28%), decreased white blood cell count (27%), increased lactate dehydrogenase (23%), musculoskeletal pain (22%), decreased appetite (20%), increased ALT (20%), and rash (20%).
Clinically relevant adverse reactions occurring in <10% of patients who received DATROWAY included dry skin, blurred vision, abdominal pain, conjunctivitis, dry mouth, ILD/pneumonitis, skin hyperpigmentation, increased lacrimation, and visual impairment.
Unresectable or Metastatic, HR-Positive, HER2-Negative Breast Cancer
TROPION-Breast01
The safety of DATROWAY was evaluated in 360 patients with unresectable or metastatic HR-positive, HER2-negative (IHC 0, IHC1+ or IHC2+/ISH-) breast cancer who received at least one dose of DATROWAY 6 mg/kg in TROPION-Breast01. DATROWAY was administered by intravenous infusion once every three weeks. The median duration of treatment was 6.7 months (range: 0.7 months to 16.1 months) for patients who received DATROWAY.
Serious adverse reactions occurred in 15% of patients who received DATROWAY. Serious adverse reactions in >0.5% of patients who received DATROWAY were urinary tract infection (1.9%), COVID-19 infection (1.7%), ILD/pneumonitis (1.1%), acute kidney injury, pulmonary embolism, vomiting, diarrhea, hemiparesis, and anemia (0.6% each). Fatal adverse reactions occurred in 0.3% of patients who received DATROWAY and were due to ILD/pneumonitis.
Permanent discontinuation of DATROWAY due to an adverse reaction occurred in 3.1% of patients. Adverse reactions which resulted in permanent discontinuation of DATROWAY in >0.5% of patients included ILD/pneumonitis (1.7%) and fatigue (0.6%).
Dosage interruptions of DATROWAY due to an adverse reaction occurred in 22% of patients. Adverse reactions which required dosage interruption in >1% of patients included COVID-19 (3.3%), infusion-related reaction (1.4%), ILD/pneumonitis (1.9%), stomatitis (1.9%), fatigue (1.7%), keratitis (1.4%), acute kidney injury (1.1%), and pneumonia (1.1%).
Dose reductions of DATROWAY due to an adverse reaction occurred in 23% of patients. Adverse reactions which required dose reduction in >1% of patients included stomatitis (13%), fatigue (3.1%), nausea (2.5%), and weight decrease (1.9%).
The most common (≥20%) adverse reactions, including laboratory abnormalities, were stomatitis (59%), nausea (56%), fatigue (44%), decreased leukocytes (41%), decreased calcium (39%), alopecia (38%), decreased lymphocytes (36%), decreased hemoglobin (35%), constipation (34%), decreased neutrophils (30%), dry eye (27%), vomiting (24%), increased ALT (24%), keratitis (24%), increased AST (23%), and increased alkaline phosphatase (23%).
Clinically relevant adverse reactions occurring in <10% of patients who received DATROWAY included infusion-related reactions (including bronchospasm), ILD/pneumonitis, headache, pruritus, dry skin, dry mouth, conjunctivitis, blepharitis, meibomian gland dysfunction, blurred vision, increased lacrimation, photophobia, visual impairment, skin hyperpigmentation, and madarosis.
Use in Specific Populations
Pregnancy: Based on its mechanism of action, DATROWAY can cause embryo-fetal harm when administered to a pregnant woman because the topoisomerase inhibitor component of DATROWAY, DXd, is genotoxic and affects actively dividing cells. There are no available data on the use of DATROWAY in pregnant women to inform a drug-associated risk. Advise patients of the potential risks to a fetus.
Lactation: There are no data regarding the presence of datopotamab deruxtecan-dlnk or its metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with DATROWAY and for 1 month after the last dose.
Females and Males of Reproductive Potential: Pregnancy Testing: Verify pregnancy status of females of reproductive potential prior to initiation of DATROWAY. Contraception: Females: Advise females of reproductive potential to use effective contraception during treatment with DATROWAY and for 7 months after the last dose. Males: Because of the potential for genotoxicity, advise male patients with female partners of reproductive potential to use effective contraception during treatment with DATROWAY and for 4 months after the last dose. Infertility: Based on findings in animal toxicity studies, DATROWAY may impair male and female reproductive function and fertility. The effects on reproductive organs in animals were irreversible.
Pediatric Use: Safety and effectiveness of DATROWAY have not been established in pediatric patients.
Geriatric Use: Of the 125 patients with EGFR-mutated NSCLC in TROPION-Lung05, TROPION-Lung01, TROPION-PanTumor01 treated with DATROWAY 6 mg/kg, 44% were ≥65 years of age and 10% were ≥75 years of age. No clinically meaningful differences in efficacy and safety were observed between patients ≥65 years of age versus younger patients. Of the 365 patients in TROPION-Breast01 treated with DATROWAY 6 mg/kg, 25% were ≥65 years of age and 5% were ≥75 years of age. Grade ≥3 and serious adverse reactions were more common in patients ≥65 years (42% and 25%, respectively) compared to patients <65 years (33% and 15%, respectively). In TROPION-Breast01, no other meaningful differences in safety or efficacy were observed between patients ≥65 years of age versus younger patients.
Renal Impairment: A higher incidence of ILD/pneumonitis has been observed in patients with mild and moderate renal impairment (creatinine clearance [CLcr] 30 to <90 mL/min). Monitor patients with renal impairment for increased adverse reactions, including respiratory reactions. No dosage adjustment is recommended in patients with mild to moderate renal impairment. The effect of severe renal impairment (CLcr <30 mL/min) on the pharmacokinetics of datopotamab deruxtecan-dlnk or DXd is unknown.
Hepatic Impairment: No dosage adjustment is recommended in patients with mild hepatic impairment (total bilirubin ≤ULN and any AST >ULN or total bilirubin >1 to 1.5 times ULN and any AST). Limited data are available in patients with moderate hepatic impairment (total bilirubin >1.5 to 3 times ULN and any AST). Monitor patients with moderate hepatic impairment for increased adverse reactions. The recommended dosage of DATROWAY has not been established for patients with severe hepatic impairment (total bilirubin >3 times ULN and any AST).
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or fda.gov/medwatch.
Please see accompanying full Prescribing Information, including the Medication Guide.
About Daiichi Sankyo
Daiichi Sankyo is an innovative global healthcare company contributing to the sustainable development of society that discovers, develops and delivers new standards of care to enrich the quality of life around the world. With more than 120 years of experience, Daiichi Sankyo leverages its world-class science and technology to create new modalities and innovative medicines for people with cancer, cardiovascular and other diseases with high unmet medical need. For more information, please visit www.daiichisankyo.com.
References
1 World Health Organization. Global Cancer Observatory: Bladder. Accessed October 2025.
2 National Library of Medicine. Bladder Cancer. Accessed October 2025.
3 National Cancer Institute. SEER Cancer Statistics Factsheets: Bladder Cancer. Accessed October 2025.
4 Saginala K, et al. Med Sci (Basel). 2020;8(1):15. Published 2020 Mar 13. doi:10.3390/medsci8010015
5 V. Thomas, et al. JAMA Network Open. 2024:2;7(5):e249417.
6 M. Abbas, et al. Oncol Lett. 2023:26(6):527.
7 C. Avellini, et al. Oncotarget. 2017 Apr 25;8(35):58642–58653.
Phase 2Clinical ResultPhase 3License out/inDrug Approval
100 Deals associated with Patritumab
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | Patritumab | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| metastatic non-small cell lung cancer | Phase 3 | United States | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Belgium | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Canada | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Czechia | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Germany | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Hungary | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Italy | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Poland | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | Spain | 01 Mar 2014 | |
| metastatic non-small cell lung cancer | Phase 3 | United Kingdom | 01 Mar 2014 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1/2 | 222 | tdswhvwwbt(ybdgklmnhs) = rwgmgazpkb bsdnjezhwb (dnvzngstjb, wiajbfmhcn - isdfzqgfxn) View more | - | 16 Jun 2021 | |||
tdswhvwwbt(ybdgklmnhs) = zcluanrsjv bsdnjezhwb (dnvzngstjb, ashzsmvzgt - rbklldmiwc) View more | |||||||
Phase 2 | - | funvgjxjvn(zispyuezlr) = awtqwiqgog gviddfvmgw (tshkixbnlz ) View more | Negative | 01 Dec 2019 | |||
Placebo | funvgjxjvn(zispyuezlr) = jhrhpllqix gviddfvmgw (tshkixbnlz ) View more | ||||||
Phase 1 | Squamous Cell Carcinoma of Head and Neck First line | 15 | txjlpffmzk(umvzlkdeza) = jviwybfjsl gefevmwgwu (muyzulidii ) View more | Positive | 15 Jan 2019 | ||
Phase 2 | 87 | (Patritumab) | bgpsemkxvx(kewkqipeba) = rtgimvaelz htjdsgpzne (vqpqnuvily, vbolicyfhi - riilcsiynz) View more | - | 07 Jan 2019 | ||
(Placebo) | bgpsemkxvx(kewkqipeba) = luyezbmctj htjdsgpzne (vqpqnuvily, lpratovcxb - qfturdbyis) View more | ||||||
Phase 3 | 145 | Placebo+Erlotinib (Placebo + Erlotinib) | blweephqjt = hbjekeaikv jguglrgygq (ahmmgpeqpw, oawbuvmnzt - ykcodnmtyv) View more | - | 23 Jan 2018 | ||
(Patritumab + Erlotinib) | blweephqjt = boeenoykpi jguglrgygq (ahmmgpeqpw, ucofxksdnd - ezyjhsplbt) View more | ||||||
Phase 2 | 212 | lavkofbcxu(gyzeymplqt): HR = 0.41 (95% CI, 0.18 - 0.9), P-Value = 0.02 | Positive | 20 May 2016 | |||
Placebo + Erlotinib | |||||||
Phase 1/2 | 145 | yqqqdykcjh(qkwyqtkjyr) = tqkcavizox irwzyxsgxf (gzkejslnha ) View more | - | 20 May 2014 | |||
yqqqdykcjh(qkwyqtkjyr) = ceztowubjg irwzyxsgxf (gzkejslnha ) View more | |||||||
Phase 2 | 215 | odsaptjoqr(gpncqgndsa) = focimqloxn chxuhztvdk (wkhviliygj ) | - | 20 May 2014 | |||
odsaptjoqr(gpncqgndsa) = rxfpcrurww chxuhztvdk (wkhviliygj ) |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
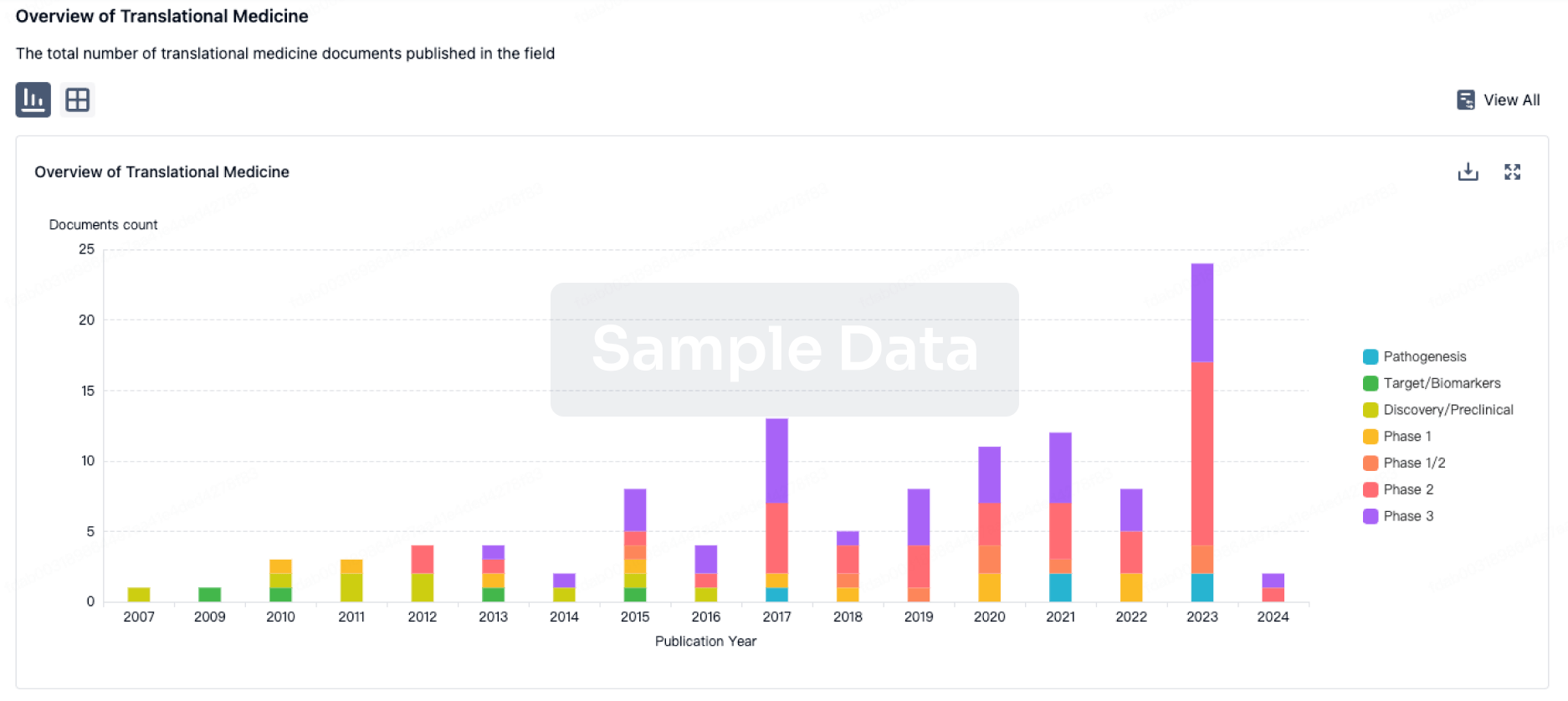
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free



