Last update 24 Jun 2024
Heptavalent combination vaccine(GlaxoSmithKline Plc)
Last update 24 Jun 2024
Overview
Basic Info
Drug Type Prophylactic vaccine |
Synonyms Heptavalent combination vaccine, Heptavalent conjugated vaccine, GSK 2202083A + [2] |
Target- |
Mechanism Immunostimulants |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Related
4
Clinical Trials associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)Immunogenicity and Safety Study of GlaxoSmithKline Biologicals' GSK2202083A Vaccine Administered as a Booster Dose in 12-18 Months Old Healthy Children
The current trial will evaluate the safety and immunogenicity of GSK Biologicals' GSK2202083A vaccine when administered as a booster dose following priming in the first year of life with the same vaccine.
This protocol posting deals with objectives & outcome measures of the booster phase. The objectives & outcome measures of the primary phase are presented in a separate protocol posting (NCT number = NCT00970307).
This protocol posting deals with objectives & outcome measures of the booster phase. The objectives & outcome measures of the primary phase are presented in a separate protocol posting (NCT number = NCT00970307).
Start Date18 Aug 2010 |
Sponsor / Collaborator |
Feasibility Study of GlaxoSmithKline Biologicals' GSK2202083A Vaccine in Healthy Infants at 2, 4 and 12 Months of Age
This study will evaluate the safety and immunogenicity of GSK Biologicals' GSK2202083 vaccine co-administered with Prevenar 13® at 2, 4 and 12 months of age and with Rotarix™ at 2 and 4 months of age.
Start Date17 May 2010 |
Sponsor / Collaborator |
Immunogenicity and Safety Study of GlaxoSmithKline Biologicals' GSK2202083A Vaccine in Healthy Infants at 2, 3 and 4 Months of Age
This study will evaluate the safety and immunogenicity of GSK Biologicals' GSK2202083A vaccine co-administered with GSK Biologicals' 10-valent pneumococcal conjugate (GSK1024850A) vaccine given as a three-dose primary vaccination course at 2, 3 and 4 months of age.
Start Date13 Aug 2009 |
Sponsor / Collaborator |
100 Clinical Results associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
100 Translational Medicine associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
100 Patents (Medical) associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
458
Literatures (Medical) associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)01 Feb 2023·Archives of virology
Assessment of the potency and effectiveness of a heptavalent oil-adjuvanted (ISA 206) foot-and-mouth disease vaccine in Egypt
Article
Author: Amer, Haitham M ; Nayel, Mohamed ; Wasfy, Momtaz ; Attia, Mohamed ; Badr, Yassien ; Bazid, Abdel-Hamid ; Magouz, Asmaa ; El-Sayed, Magdy M ; Maklad, Nada ; Abdelmegeid, Mohamed
Abstract:
Foot-and-mouth disease (FMD) is a serious highly contagious viral disease affecting all cloven-hoofed animals, and outbreaks can have a severe economic impact. An inactivated heptavalent oil-adjuvanted FMD vaccine (Aphtovac-7, MEVAC) was prepared from the foot-and-mouth disease virus (FMDV) strains A-Iran05, A-Africa-IV, O-PanAsia2, O-Manisa, O-EA3, SAT-2 Gharbia, and SAT-2 LIB-12. The vaccine potency and effectiveness were evaluated in three groups of 6- to 8-month-old calves and 200 adult dairy cattle under field conditions. All animals were vaccinated with the vaccine preparation, and the three groups of calves were challenged after 28 days by intradermolingual inoculation with 104 50% tissue culture infective dose (TCID50) of FMDV serotype A, O, or SAT-2. Mock-vaccinated calves (two per group) served as unvaccinated controls during the challenge test. Adult dairy cattle were tested for seroconversion using a virus neutralization test at 30, 60, and 120 days post-vaccination. All calves displayed complete protection against challenge with the different serotypes of FMDV when compared to the control groups. Serum samples collected after the primary and booster immunizations at 30 days post-vaccination contained high titers of protective antibodies (≥ 1/32; i.e. 1.5 log10). Antibodies persisted until the end of the study period (120 days), with a peak value around 60 days post-vaccination. The heptavalent FMD vaccine preparation was found to be potent and capable of providing a protective immune response under both experimental and field conditions.
01 Jul 2019·Revista Argentina de microbiologiaQ4 · BIOLOGY
Clinical and microbiological characteristics of community-acquired pneumonia associated with Streptococcus pneumoniae in adult patients in Mexico
Q4 · BIOLOGY
ArticleOA
Author: Soto-Nogueron, Araceli ; Rodríguez-Noriega, Eduardo ; Carnalla-Barajas, Maria Noemí ; Morfín-Otero, Rayo ; Román-Mancha, Alma Lucía ; Camacho-Ortiz, Adrián ; Garza-González, Elvira ; Guajardo-Lara, Claudia Elena ; Ayala-Gaytán, Juan Jacobo ; Echaniz-Aviles, Gabriela
The aim of this study was to assess the risk factors and clinical and microbiological characteristics of community-acquired pneumonia (CAP) in adult patients in Mexico. Streptococcus pneumoniae classified as the causative agent of CAP in adult patients and patients with invasive S. pneumoniae isolates presented to three tertiary teaching hospitals during the 15-year study period were selected. Serotyping and susceptibility testing were performed for all included isolates. Clinical and demographic data were recorded. A total of 96 patients infected with S. pneumoniae (71 with CAP, 25 with invasive disease) were included. The CAP group involved more males (74.6%) than the invasive disease group (p=0.03). Head trauma was more common in the CAP group (21.1%) than in the invasive disease group (4.0%; p=0.03). The most prevalent serotype was 19A, followed by serotypes 3 and 23F. After the introduction of the heptavalent conjugated pneumococcal vaccine (PCV7), the prevalence of included serotypes declined significantly; no such change was found after the introduction of the PCV13 vaccine, including in the prevalence of serotype 19A. Susceptibility to all antimicrobials tested except vancomycin declined over the study period. In conclusion, head trauma was the most common comorbidity in the CAP group. The most prevalent serotype was 19A. Decreased susceptibility to most antimicrobials tested was observed.
01 Aug 2018·VaccineQ3 · MEDICINE
Safety and immunogenicity of the Cuban heptavalent pneumococcal conjugate vaccine in healthy infants. Results from a double-blind randomized control trial Phase I
Q3 · MEDICINE
Article
Author: Yarisset Ricardo Delgado ; Yury Valdés-Balbín ; Dagmar García-Rivera ; Beatriz Paredes Moreno ; Laura M. Rodriguez-Noda ; Yanet Rodríguez ; Vicente Vérez-Bencomo ; María E. Mesa ; Nadezhda González ; Dania Vega ; Darielys Santana Mederos ; Vladimir Echemendía ; Nivaldo Linares-Pérez ; José A. Broche ; David Goldblatt ; Rafael del Valle-Rodríguez ; Marlene Armesto ; Carlos P. Dotres Martínez ; Carmen R. Ruiz ; Amarilis Pérez ; Rinaldo Puga Gómez ; Mayelín Mirabal Sosa ; Ada Rodríguez-Concepción ; Pablo Cabrera ; Marisel Martínez ; Yarmila García ; Annaliet Iglesias ; María E. Toledo-Romaní ; Alina Álvarez ; Nayibi Nuñez
BACKGROUND:
Cuba has a new pneumococcal conjugate vaccine candidate (PCV7-TT). This study evaluates the safety and immunogenicity in healthy infants using 2p+1 vaccination schedule.
METHODS:
A phase I, controlled, randomized and double blind clinical trial was designed. 30 unvaccinated healthy infants were included. 20 subjects were assigned to study group (PCV7-TT) and 10 to control group (Synflorix®) to receive the vaccines at 7, 8 months of age (primary series) and 11 months (booster dose). Blood samples were collected 30 days after second dose and post booster for antibodies measure analysis by ELISA and OPA. The statistics analysis included the frequency of occurrence for adverse events and the immune response. Non-parametric tests were used to compare the immune response. The clinical trial was published in the Cuban Public Register of Clinical Trials with code RPCEC00000173 available at http://registroclinico.sld.cu.
RESULTS:
Overall, the safety profile of PCV7-TT was similar to Synflorix®. Local reactions were predominant and systemic events were mild in severity. Swelling and redness were frequently associated with PCV7-TT mainly after the first dose (50% and 40% respectively). 15% and 10% of subject reported severe swelling after first dose with PCV7-TT and after second dose with Synflorix®. Mild fever (≥38-≤39), vomiting and sleep disturb were the systemic events reported. 100% of infants achieved pneumococcal IgG antibody concentrations ≥0.35 µg/ml after booster dose for serotypes 1, 14, 18C and 19F in each vaccine group. For serotypes 5, 6B and 23F, more than 80% infants vaccinated with Synflorix® or PCV7-TT achieved protective IgG GMC ≥ 0.35 µg/ml after booster dose. OPA proportion's responders to the seven common serotypes were 89.5% or more after the primary dose and 100% after booster dose in vaccinated with PCV7-TT.
CONCLUSIONS:
The Cuban PCV7-TT is safe, well tolerated and immunogenic in healthy infants.
2
News (Medical) associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)06 Mar 2024
Fourth Quarter 2023 Total Revenues of $277 million, which aligned our Full Year to the mid-point of guidance
Full Year 2023 Total Revenues of $1.05 billion, which was the mid-point of guidance
Fourth Quarter 2023 Net Loss of $50 million and Adjusted EBITDA of $3 million
Issues Q1 2024 and FY 2024 guidance
GAITHERSBURG, Md., March 06, 2024 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE: EBS) today reported financial results for the quarter and year ended December 31, 2023.
"Emergent has a long history of helping protect people around the world from opioid overdose emergencies and chemical, biological and radiological threats. This commitment to public health, together with Emergent's leadership, give me confidence in the long-term future of the company," said Joe Papa, President and CEO at Emergent. "Emergent faces some short-term challenges, which we are addressing head on. At the same time, we are making decisions and putting strategies in place for Emergent to add value for customers, patients and investors. Emergent is a company with a bright future, and I am excited to help lead it forward."
FINANCIAL HIGHLIGHTS (1)
Q4 2023 vs. Q4 2022
($ in millions, except per share amounts)
Q4 2023
Q4 2022
% Change
Total Revenues
$
276.6
$
330.2
(16)%
Net Loss
$
(49.5
)
$
(67.0
)
26
%
Net Loss per Diluted Share
$
(0.95
)
$
(1.34
)
29
%
Adjusted Net Income (Loss) (2)
$
(40.0
)
$
5.9
*
Adjusted Net Income (Loss) per Diluted Share (2)
$
(0.77
)
$
0.11
*
Adjusted EBITDA (2)
$
3.4
$
44.0
(92)%
Total Segment Gross Margin % (2)
31
%
32
%
Total Segment Adjusted Gross Margin %(2)
32
%
48
%
* % change is greater than +/- 100%
Full Year 2023 vs. Full Year 2022
($ in millions, except per share amounts)
2023
2022
% Change
Total Revenues
$
1,049.3
$
1,117.5
(6)%
Net Loss
$
(760.5
)
$
(211.6
)
*
Net Loss per Diluted Share
$
(14.85
)
$
(4.22
)
*
Adjusted Net Loss (2)
$
(319.0
)
$
(99.7
)
*
Adjusted Net Loss per Diluted Share (2)
$
(6.23
)
$
(1.98
)
*
Adjusted EBITDA (2)
$
(22.3
)
$
28.8
*
Total Segment Gross Margin % (2)
31
%
36
%
Total Segment Adjusted Gross Margin %(2)
33
%
41
%
* % change is greater than +/- 100%
SELECT Q4 2023 AND FULL YEAR BUSINESS UPDATES
Launched NARCAN® Naloxone HCl Nasal Spray 4 mg Over-The-Counter (“OTC NARCAN®”), broadening our customer base and sales channels to retail pharmacies and digital commerce websites as well as through physician-directed or standing order prescriptions at retail pharmacies, health departments, local law enforcement agencies, community-based organizations, substance abuse centers and other federal agencies
Announced U.S. Food and Drug Administration (“FDA”) approval of CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted), previously known as AV7909, a two-dose anthrax vaccine for post-exposure prophylaxis use
Awarded a $75 million option to Emergent's existing contract for the acquisition of the newly approved CYFENDUS®
Awarded a 10-year contract by the Biomedical Advanced Research and Development Authority (“BARDA”) for advanced development, manufacturing scale-up, and procurement of Ebanga™ (ansuvimab-zykl) product, a treatment for Ebola
Awarded a $379.6 million U.S. Department of Defense contract for RSDL®
The FDA closed out its inspection of the Company’s Camden facility and issued a “close-out letter” of its Warning Letter issued in August 2022
Continued progress on strengthening our fundamentals with key focus on our Medical Countermeasure (“MCM”) and NARCAN® products
Implemented organizational and resource changes resulting in $160 million of annual savings
Amended and extended maturity of our secured credit facility to May 2025
Divested the Travel Health business valued at $380 million
FOURTH QUARTER 2023 FINANCIAL PERFORMANCE (1)
Revenues
Beginning in 2023, the Company revised the categories used in discussing product/service level revenues. The new categories are:
NARCAN® — comprises contributions from NARCAN® Nasal Spray
Other Commercial Products - comprises contributions from Vaxchora® and Vivotif®, which we sold to Bavarian Nordic as part of our travel health business in May 2023
Anthrax MCM — comprises potential contributions from CYFENDUS®, previously known as AV7909, BioThrax®, Anthrasil® and Raxibacumab
Smallpox MCM — comprises potential contributions from ACAM2000®, VIGIV and TEMBEXA®
Other Products — comprises potential contributions from BAT®, RSDL® and Trobigard®
Bioservices — comprises service and lease revenues from the Bioservices business
($ in millions)
Q4 2023
Q4 2022
% Change
Product sales, net (3):
NARCAN®
$
111.0
$
91.1
22
%
Other Commercial Products
—
4.9
NM
Anthrax MCM
111.6
56.4
98
%
Smallpox MCM
11.5
144.6
(92)%
Other Products
15.0
8.7
72
%
Total Product sales, net
$
249.1
$
305.7
(19)%
Bioservices:
Services
$
20.6
$
17.2
20
%
Leases
0.2
0.2
—
%
Total Bioservices revenues
$
20.8
$
17.4
20
%
Contracts and grants
$
6.7
$
7.1
(6)%
Total revenues
$
276.6
$
330.2
(16)%
NM - Not Meaningful
Products Revenue, net
NARCAN®
For Q4 2023, revenues from NARCAN® (naloxone HCl) Nasal Spray increased $19.9 million, or 22%, as compared with Q4 2022. The increase was primarily driven by higher OTC NARCAN® sales to U.S. public interest channels and retailers, partially offset by a decrease in sales of prescription based NARCAN® due to the launch of OTC NARCAN® in 2023 and the cessation of authorized generic NARCAN® sales related to the termination of the Company’s relationship with Sandoz.
Other Commercial Products
For Q4 2023, revenues from Other Commercial Products decreased $4.9 million as compared with Q4 2022. The decrease was driven by no sales of our Vaxchora® and Vivotif® products during the current year quarter, which we sold to Bavarian Nordic as part of our travel health business in May 2023.
Anthrax MCM
For Q4 2023, revenues from Anthrax MCM increased $55.2 million, or 98%, as compared with Q4 2022. The increase reflects the impact of timing of sales related to CYFENDUS® (Anthrax Vaccine Adsorbed, Adjuvanted), Anthrasil® (Anthrax Immune Globulin Intravenous (human)) and BioThrax® (Anthrax Vaccine Adsorbed). Anthrax vaccine product sales are primarily made under annual purchase options exercised by the U.S. Government ("USG"). Fluctuations in revenues result from the timing of the exercise of annual purchase options, the timing and amount of USG purchases, the availability of governmental funding and timing of Company delivery of orders that follow.
Smallpox MCM
For Q4 2023, revenues from Smallpox MCM decreased $133.1 million, or 92%, as compared with Q4 2022. The decrease was due to no current quarter sales of TEMBEXA® and timing of VIGIV deliveries. Fluctuations in revenues from Smallpox MCM result from the timing of the exercise of annual purchase options in existing procurement contracts, the timing of USG purchases, the availability of governmental funding and timing of Company delivery of orders that follow.
Other Products
For Q4 2023, revenues from other product sales increased $6.3 million, or 72%, as compared with Q4 2022. The increase was primarily due to higher BAT® product sales, partially offset by lower sales of RSDL®.
Bioservices Revenues
Services
For Q4 2023, revenues from Bioservices services increased $3.4 million, or 20%, as compared with Q4 2022. The increase was primarily driven by resolution of a customer's outstanding obligation at our Bayview facility and increased production at our Camden facility, partially offset by a decrease in production at our Winnipeg facility. In the prior year, there was a reversal of revenue related to the halt in manufacturing under the Janssen Agreement.
Leases
For Q4 2023, revenues from Bioservices leases were consistent with Q4 2022.
Contracts and Grants
For Q4 2023, revenues from contracts and grants decreased $0.4 million, or 6%, as compared with Q4 2022. The decrease was primarily attributable to changes in the mix and timing of various development initiatives.
Operating Expenses
($ in millions)
Q4 2023
Q4 2022
% Change
Cost of Commercial product sales
$
50.1
$
40.2
25
%
Cost of MCM product sales
97.2
127.6
(24)%
Cost of Bioservices
37.8
52.7
(28)%
Goodwill impairment
—
6.7
NM
Research and development (“R&D”)
29.4
47.0
(37)%
Selling, general and administrative (“SG&A”)
89.7
93.4
(4)%
Amortization of intangible assets
16.2
17.9
(9)%
Total operating expenses
$
320.4
$
385.5
(17)%
NM - Not Meaningful
Cost of Commercial Product Sales
For Q4 2023, cost of Commercial product sales increased $9.9 million, or 25%, as compared with Q4 2022. The increase was primarily due to higher sales of OTC NARCAN® and Branded NARCAN®, partially offset by no sales of Vivotif® and Vaxchora® or related expenses during the current quarter due to the sale of our travel health business to Bavarian Nordic in May 2023.
Cost of MCM Product Sales
For Q4 2023, cost of MCM product sales decreased $30.4 million, or 24%, as compared with Q4 2022. The decrease was primarily due to lower sales of TEMBEXA® and lower shutdown costs, partially offset by increases due to higher sales of CYFENDUS®, Anthrasil® and BioThrax®, inventory write-offs and additional allocations of cost of goods sold to MCM Products from Bioservices.
Cost of Bioservices
For Q4 2023, cost of Bioservices decreased $14.9 million, or 28%, as compared with Q4 2022. The decrease was primarily due to higher allocations to MCM Product cost of goods sold and reduced production activities related to the halt in manufacturing under the Janssen Agreement at our Bayview facility, coupled with decreases in production at the Company’s Camden and Winnipeg facilities.
Research and Development Expenses
For Q4 2023, R&D expenses decreased $17.6 million, or 37%, as compared with Q4 2022. The decrease was primarily due to the sale of the Company’s development program for CHIKV VLP to Bavarian Nordic, which was a significant contributor to prior period R&D expense, as well as a decrease in funded R&D across various development initiatives and reduction in related overhead costs driven by headcount reductions, partially offset by write-offs related to program terminations during the period and an increase in Ebanga™ and TEMBEXA® funded R&D.
Selling, General and Administrative Expenses
For Q4 2023, SG&A expenses decreased $3.7 million, or 4%, as compared with Q4 2022. The decrease was primarily due to decreases in consulting and contracted services and decreases in compensation and other employee costs related to the restructuring initiatives taken during 2023. The decrease was partially offset by an increase in marketing expenses related to the launch of OTC NARCAN® and higher professional services fees related to legal remediation services.
Goodwill Impairment
For Q4 2023, goodwill impairment decreased $6.7 million as compared with Q4 2022. The decrease was related to the Q4 2022 $6.7 million non-cash impairment charge to Goodwill in the Bioservices reporting unit, which reduced the reporting unit's goodwill balance to zero.
ADDITIONAL FINANCIAL INFORMATION (1)
Capital Expenditures
($ in millions)
Q4 2023
Q4 2022
% Change
Capital expenditures
$
11.4
$
23.6
(52)%
Less: capital expenditures reimbursed
—
2.5
NM
Net capital expenditures
$
11.4
$
21.1
(46)%
Capital expenditures as a % of total revenues
4
%
7
%
Net capital expenditures as a % of total revenues
4
%
6
%
NM - Not Meaningful
For Q4 2023, capital expenditures decreased largely due to lower product development activities across the Company’s facilities.
SEGMENT INFORMATION
In the fourth quarter of 2023, we realigned our reportable operating segments to reflect recent changes in our internal operating and reporting process. The Company now manages the business with a focus on three reportable segments: (1) a Commercial Products segment consisting of our NARCAN® and other commercial products which were sold as part of our travel health business in the second quarter of 2023; (2) a MCM Products segment consisting of the Anthrax - MCM, Smallpox - MCM and Other products and (3) a services segment (“Services”) consisting of our Bioservices. The Company evaluates the performance of these reportable segments based on revenue and segment adjusted gross margin, which is a non-GAAP financial measure. Segment revenue includes external customer sales, but does not include inter-segment services. The Company does not allocate contracts and grants, R&D, SG&A, amortization of intangible assets, interest and other income (expense) or taxes to its evaluation of the performance of these segments.
FOURTH QUARTER 2023 SEGMENT RESULTS
($ in millions)
Commercial Products
Quarter Ended December 31,
2023
2022
$ Change
% Change
Revenues
$
111.0
$
96.0
$
15.0
16
%
Cost of sales
50.1
40.2
9.9
25
%
Gross margin **
$
60.9
$
55.8
$
5.1
9
%
Gross margin % **
55
%
58
%
Segment adjusted gross margin (2)
$
60.9
$
55.8
$
5.1
9
%
Segment adjusted gross margin % (2)
55
%
58
%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
Commercial Products gross margin increased $5.1 million, or 9%, to $60.9 million in the quarter, as compared with $55.8 million in the prior year quarter. Commercial Products gross margin percentage decreased 3 percentage points to 55% for the quarter ended December 31, 2023. The decrease was primarily due to an increase in royalty expense on OTC NARCAN® compared to Q4 2022, partially offset by a favorable change in product mix related to the sale of our travel health products. Commercial Products segment adjusted gross margin was consistent with gross margin.
($ in millions)
MCM Products
Quarter Ended December 31,
2023
2022
$ Change
% Change
Revenues
$
138.1
$
209.7
$
(71.6
)
(34)%
Cost of sales
97.2
127.6
(30.4
)
(24)%
Gross margin **
$
40.9
$
82.1
$
(41.2
)
(50)%
Gross margin % **
30
%
39
%
Add back:
Changes in fair value of contingent consideration
$
0.6
$
0.2
$
0.4
*
Inventory step-up provision
2.0
51.4
(49.4
)
(96)%
Restructuring costs
(1.4
)
—
(1.4
)
NM
Segment adjusted gross margin (2)
$
42.1
$
133.7
$
(91.6
)
(69)%
Segment adjusted gross margin % (2)
30
%
64
%
* % change is greater than +/- 100%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
NM - Not Meaningful
MCM Products gross margin decreased $41.2 million, or 50%, to $40.9 million in the quarter, as compared with $82.1 million in the prior year quarter. MCM Products gross margin percentage decreased 9 percentage points to 30% for the quarter ended December 31, 2023. The decrease was largely due to lower sales volumes and inventory write-offs, coupled with an unfavorable product revenue mix which was weighted more heavily to lower margin products compared with the prior year quarter. MCM Product segment adjusted gross margin in the current year period excludes the impact of non-cash items related to the changes in the fair value of contingent consideration of $0.6 million, the inventory step-up provision of $2.0 million and the impact of restructuring costs of $(1.4) million.
($ in millions)
Services
Quarter Ended December 31,
2023
2022
$ Change
% Change
Revenues
$
20.8
$
17.4
$
3.4
20
%
Cost of services
37.8
52.7
(14.9
)
(28)%
Gross margin **
$
(17.0
)
$
(35.3
)
$
18.3
52
%
Gross margin % **
(82
)%
(203
)%
Add back:
Restructuring costs
$
0.3
$
—
$
0.3
NM
Segment adjusted gross margin (2)
$
(16.7
)
$
(35.3
)
$
18.6
53
%
Segment adjusted gross margin % (2)
(80
)%
(203
)%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
NM - Not Meaningful
Services gross margin increased $18.3 million, or 52%, to $(17.0) million in the quarter, as compared with $(35.3) million in the prior year quarter. Services gross margin percentage increased 121 percentage points to (82)% for the quarter ended December 31, 2023. The increase was primarily due to the resolution of a customer's outstanding obligation at our Bayview facility coupled with one-time costs and reserves related to the Janssen Agreement in the prior year quarter, partially offset by additional investments in quality enhancement and improvement initiatives at the Company's Camden facility and decreased production at the Company's Winnipeg facility in the current year. Services segment adjusted gross margin in the current year period excludes the impact of restructuring costs of $0.3 million.
FULL YEAR 2023 SEGMENT RESULTS
($ in millions)
Commercial Products
Year Ended December 31,
2023
2022
$ Change
% Change
Revenues
$
497.3
$
386.6
$
110.7
29
%
Cost of sales
210.3
160.3
50.0
31
%
Gross margin **
$
287.0
$
226.3
$
60.7
27
%
Gross margin % **
58
%
59
%
Segment adjusted gross margin (2)
$
287.0
$
226.3
$
60.7
27
%
Segment adjusted gross margin % (2)
58
%
59
%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
Commercial Products gross margin increased $60.7 million, or 27%, to $287.0 million in 2023, as compared with $226.3 million in the prior year. Commercial Products gross margin percentage decreased 1 percentage point to 58% in 2023. The decrease was largely due to a decrease in the per unit selling price in response to increased competition for generic NARCAN®, partially offset by a decrease in royalty expense. Commercial Products segment adjusted gross margin was consistent with gross margin.
($ in millions)
MCM Products
Year Ended December 31,
2023
2022
$ Change
% Change
Revenues
$
447.2
$
579.6
$
(132.4
)
(23)%
Cost of sales
305.6
264.3
41.3
16
%
Gross margin **
$
141.6
$
315.3
$
(173.7
)
(55)%
Gross margin % **
32
%
54
%
Add back:
Changes in fair value of contingent consideration
$
0.2
$
2.6
$
(2.4
)
(92)%
Inventory step-up provision
3.9
51.4
(47.5
)
(92)%
Restructuring costs
5.6
—
5.6
NM
Segment adjusted gross margin (2)
$
151.3
$
369.3
$
(218.0
)
(59)%
Segment adjusted gross margin % (2)
34
%
64
%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
NM - Not Meaningful
MCM Products gross margin decreased $173.7 million, or 55%, to $141.6 million in 2023, as compared with $315.3 million in the prior year. MCM Products gross margin percentage decreased 22 percentage points to 32% for the year ended December 31, 2023. The decrease was largely due to lower sales volumes and higher shutdown related costs and inventory write-offs, coupled with an unfavorable product revenue mix which was weighted more heavily to lower margin products compared with the prior year. MCM Product segment adjusted gross margin in 2023 excludes the impact of restructuring costs of $5.6 million, non-cash items related to the changes in the fair value of contingent consideration of $0.2 million and the inventory step-up provision of $3.9 million.
($ in millions)
Services
Year Ended December 31,
2023
2022
$ Change
% Change
Revenues
$
78.5
$
109.9
$
(31.4
)
(29)%
Cost of services
189.5
268.5
(79.0
)
(29)%
Gross margin **
$
(111.0
)
$
(158.6
)
$
47.6
30
%
Gross margin % **
(141
)%
(144
)%
Add back:
Restructuring costs
$
8.4
$
—
$
8.4
NM
Segment adjusted gross margin (2)
$
(102.6
)
$
(158.6
)
$
56.0
35
%
Segment adjusted gross margin % (2)
(131
)%
(144
)%
** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.
NM - Not Meaningful
Services gross margin increased $47.6 million, or 30%, to $(111.0) million in 2023, as compared with $(158.6) million in the prior year. Services gross margin percentage increased 3 percentage points to (141)% for the year ended December 31, 2023. The increase was primarily driven by one-time costs and reserves related to the Janssen Agreement in the prior year. Services segment adjusted gross margin in 2023 excludes the impact of restructuring costs of $8.4 million.
2024 FINANCIAL FORECAST
The Company provides the following financial forecast for full year 2024 and Q1 2024, in both instances reflecting management's expectations based on the most current information available.
Full Year 2024
($ in millions)
METRIC
Full Year 2023 Actual
Full Year 2024 Forecast
Total revenues
$1,049.3
$900 - $1,100
Net loss
$(760.5)
$(183) - $(133)
Adjusted net loss (2)
$(319.0)
$(130) - $(80)
Adjusted EBITDA (2)
$(22.3)
$50 - $100
Total segment adjusted gross margin % (2)
33%
40% - 45%
Segment Level Revenue (4)
Commercial Products
$497.3
$460 - $500
MCM Products
$447.2
$340 - $490
Services
$78.5
$70 - $80
Q1 2024
($ in millions)
METRIC
Q1 2024 Forecast
Total revenues
$200 - $250
FOOTNOTES
(1) All financial information included in this release is unaudited.
(2) See “Non-GAAP Financial Measures” and the "Reconciliation of Non-GAAP Financial Measures" tables for the definitions and reconciliations of these non-GAAP financial measures to the most closely related GAAP financial measures.
(3) Product sales, net are reported net of variable consideration including returns, rebates, wholesaler fees and prompt pay discounts in accordance with U.S. generally accepted accounting principles.
(4) Other Commercial products, which includes Vivotif® and Vaxchora®, which were sold to Bavarian Nordic as part of our travel health business in May 2023, are not included in the 2024 forecast.
CONFERENCE CALL, PRESENTATION SUPPLEMENT AND WEBCAST INFORMATION
Company management will host a conference call at 5:00 pm eastern time today, March 6, 2024, to discuss these financial results. The conference call and presentation supplement can be accessed from the Company's website or through the following:
By phone
Advance registration is required.
Visit to register and receive an email with the dial-in number, passcode and registrant ID.
By webcast
Visit
A replay of the call can be accessed from the Emergent website.
ABOUT EMERGENT BIOSOLUTIONS INC.
At Emergent, our mission is to protect and enhance life. We develop, manufacture, and deliver protections against public health threats through a pipeline of innovative vaccines and therapeutics. For over 20 years, we have been at work defending people from things we hope will never happen—so that we are prepared just in case they ever do. We do what we do because we see the opportunity to create a better, more secure world. One where preparedness empowers protection from the threats we face. And peace of mind prevails. In working together, we envision protecting or enhancing 1 billion lives by 2030. For more information, visit our website and follow us on LinkedIn, Twitter, and Instagram.
NON-GAAP FINANCIAL MEASURES
In the accompanying analysis of financial information, we sometimes use information derived from consolidated and segment financial information that may not be presented in our financial statements or prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). Certain of these financial measures are considered not in conformity with GAAP (“non-GAAP financial measures”) under the United States Securities and Exchange Commission (“SEC”) rules. Specifically, we have referred to the following non-GAAP financial measures:
Adjusted Net Income (Loss)
Adjusted Net Income (Loss) per Diluted Share
Adjusted EBITDA
Total Segment Revenues
Total Segment Gross Margin
Total Segment Gross Margin %
Total Segment Adjusted Gross Margin
Total Segment Adjusted Gross Margin %
Segment Adjusted Gross Margin
Segment Adjusted Gross Margin %
We define Adjusted Net Income (Loss) and Adjusted Net Income (Loss) per Diluted Share, which are non-GAAP financial measures, as net loss and net loss per diluted share, respectively, excluding the impact of changes in fair value of contingent consideration, acquisition and divestiture-related costs, goodwill and long-lived asset impairment charges, severance and restructuring costs, exit and disposal costs, inventory step-up provisions, and non-cash amortization charges. We use Adjusted Net Income (Loss) for the purpose of calculating Adjusted Net Income (Loss) per Diluted Share. Management uses Adjusted Net Income (Loss) per Diluted Share to assess total Company operating performance on a consistent basis. We believe that these non-GAAP financial measures, when considered together with our GAAP financial results and GAAP financial measures, provide management and investors with an additional understanding of our business operating results, including underlying trends.
We define Adjusted EBITDA, which is a non-GAAP financial measure, as consolidated net loss before income tax provision (benefit), interest expense, net, depreciation, amortization of intangible assets, changes in fair value of contingent consideration, goodwill and long-lived asset impairment charges, severance and restructuring costs, exit and disposal costs, acquisition and divestiture-related costs and inventory step-up provisions. We believe that this non-GAAP financial measure, when considered together with our GAAP financial results and GAAP financial measures, provides management and investors with a more complete understanding of our operating results, including underlying trends. In addition, EBITDA is a common alternative measure of operating performance used by many of our competitors. It is used by investors, financial analysts, rating agencies and others to value and compare the financial performance of companies in our industry, although it may be defined differently by different companies. Therefore, we also believe that this non-GAAP financial measure, considered along with corresponding GAAP financial measures, provides management and investors with additional information for comparison of our operating results with the operating results of other companies.
We have included the definitions of segment gross margin and segment gross margin %, which are GAAP financial measures, below in order to more fully define the components of certain non-GAAP financial measures presented in this press release. We define Segment Gross Margin, as a segment's revenues, less a segment's cost of sales or services. We define Segment Gross Margin %, as Segment Gross Margin as a percentage of a segments revenues. We define Segment Adjusted Gross Margin, which is a non-GAAP financial measure as Segment Gross Margin excluding the impact of restructuring costs and non-cash items related to changes in fair value of contingent consideration and inventory step-up provision. We define Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Segment Adjusted Gross Margin as a percentage of a segment's revenues.
We define Total Segment Revenues, which is a non-GAAP financial measure, as our Total Revenues, less contracts and grants revenue, which is also equal to the sum of the revenues of our operating segments. We define Total Segment Gross Margin, which is a non-GAAP financial measure, as Total Segment Revenues less our aggregate cost of sales or services. We define Total Segment Gross Margin %, which is a non-GAAP financial measure, as Total Segment Gross Margin as a percentage of Total Segment Revenues. We define Total Segment Adjusted Gross Margin, which is a non-GAAP financial measure, as Total Segment Gross Margin, excluding the impact of restructuring costs, inventory step-up provision and the fair value of contingent consideration. We define Total Segment Adjusted Gross Margin %, which is a non-GAAP financial measure, as Total Segment Adjusted Gross Martin as a percentage of Total Segment Revenues.
Non-GAAP financial measures are not defined in the same manner by all companies and may not be comparable with other similarly titled measures of other companies. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Non-GAAP financial measures should be considered in addition to, but not as a substitute for or superior to, the information contained in our Consolidated Statements of Operations and Consolidated Statements of Cash Flows. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures are included in the financial tables accompanying this press release.
SAFE HARBOR STATEMENT
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the future performance of the Company or our business strategy, future operations, future financial position, future revenues and earnings, our ability to achieve the objectives of our restructuring initiatives, including our future results, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “goal,” “intend,” “may,” “plan,” “position,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake any obligation to update any forward-looking statement to reflect new information, events or circumstances.
There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including, among others, the availability of USG funding for contracts related to procurement of our MCM products, including CYFENDUS® (Anthrax Vaccine Adsorbed (AVA), Adjuvanted), BioThrax® (Anthrax Vaccine Adsorbed), ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), among others, as well as contracts related to development of medical countermeasures; the availability of government funding for our other commercialized products, including Ebanga™ (ansuvimab-zykl), BAT® (Botulism Antitoxin Heptavalent) and RSDL® (Reactive Skin Decontamination Lotion Kit); our ability to meet our commitments to quality and compliance in all of our manufacturing operations; our ability to negotiate additional USG procurement or follow-on contracts for our MCM products that have expired or will be expiring; the commercial availability and acceptance of over-the-counter NARCAN® (naloxone HCl) Nasal Spray; the impact of the generic marketplace on NARCAN® (naloxone HCI) Nasal Spray and future NARCAN® sales; our ability to perform under our contracts with the USG, including the timing of and specifications relating to deliveries; our ability to provide Bioservices for the development and/or manufacture of product and/or product candidates of our customers at required levels and on required timelines; the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to negotiate further commitments related to the collaboration and deployment of capacity toward future commercial manufacturing under our existing Bioservices contracts; our ability to collect reimbursement for raw materials and payment of services fees from our Bioservices customers; the results of pending stockholder litigation and government investigations and their potential impact on our business; our ability to comply with the operating and financial covenants required by our senior secured credit facilities and the amended and restated credit agreement relating to such facilities, and our 3.875% Senior Unsecured Notes due 2028; our ability to resolve the going concern qualification in our consolidated financial statements and otherwise successfully manage our liquidity in order to continue as a going concern; the procurement of our product candidates by USG entities under regulatory authorities that permit government procurement of certain medical products prior to FDA marketing authorization, and corresponding procurement by government entities outside of the United States; our ability to realize the expected benefits of the sale of our travel health business to Bavarian Nordic; the impact of the organizational changes we announced in January 2023 and August 2023; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; the impact of cyber security incidents, including the risks from the unauthorized access interruption, failure or compromise of our information systems or those of our business partners, collaborators or other third parties; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Readers should consider this cautionary statement, as well as the risks identified in our periodic reports filed with the Securities and Exchange Commission, when evaluating our forward-looking statements.
Trademarks
Emergent®, BioThrax®, BaciThrax®, RSDL®, BAT®, Trobigard®, Anthrasil®, CNJ-016®, ACAM2000®, NARCAN®, CYFENDUS®, TEMBEXA® and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries. All other brands, products, services and feature names or trademarks are the property of their respective owners.
Investor Contact
Rich Lindahl
Executive Vice President, Chief Financial Officer
lindahlr@ebsi.com
Media Contact
Assal Hellmer
Vice President, Communications
mediarelations@ebsi.com
Emergent BioSolutions Inc.
Consolidated Balance Sheets
(unaudited, in millions, except per share data)
December 31,
December 31,
2023
2022
ASSETS
Current assets:
Cash and cash equivalents
$
111.7
$
642.6
Accounts receivable, net
191.0
159.2
Inventories, net
328.9
350.7
Prepaid expenses and other current assets
47.9
57.9
Total current assets
679.5
1,210.4
Property, plant and equipment, net
382.8
817.6
Intangible assets, net
566.6
728.8
Goodwill
—
218.2
Other assets
194.3
191.3
Total assets
$
1,823.2
$
3,166.3
LIABILITIES AND STOCKHOLDERS’ EQUITY
Current liabilities:
Accounts payable
$
112.2
$
103.5
Accrued expenses
18.6
34.9
Accrued compensation
74.1
87.3
Debt, current portion
413.7
957.3
Other current liabilities
32.7
45.9
Total current liabilities
651.3
1,228.9
Debt, net of current portion
446.5
448.5
Deferred tax liability
47.2
59.7
Other liabilities
28.9
41.5
Total liabilities
$
1,173.9
$
1,778.6
Stockholders’ equity:
Preferred stock, par value $0.001 per share; 15.0 shares authorized, no shares issued and outstanding
—
—
Common stock, par value $0.001 per share; 200.0 shares authorized, 57.8 and 55.7 shares issued; 52.2 and 50.1 shares outstanding, respectively.
0.1
0.1
Treasury stock, at cost, 5.6 and 5.6 common shares, respectively
(227.7
)
(227.7
)
Additional paid-in capital
904.4
873.5
Accumulated other comprehensive income (loss), net
(5.7
)
3.1
Retained earnings (accumulated deficit)
(21.8
)
738.7
Total stockholders’ equity
$
649.3
$
1,387.7
Total liabilities and stockholders’ equity
$
1,823.2
$
3,166.3
Emergent BioSolutions Inc.
Consolidated Statements of Operations
(unaudited, in millions, except per share data)
Three Months Ended December 31,
Year Ended December 31,
2023
2022
2023
2022
Revenues:
Commercial Product sales
$
111.0
$
96.0
$
497.3
$
386.6
MCM Product sales
138.1
209.7
447.2
579.6
Total Product sales, net
249.1
305.7
944.5
966.2
Bioservices:
Services
20.6
17.2
72.8
105.0
Leases
0.2
0.2
5.7
4.9
Total Bioservices revenues
20.8
17.4
78.5
109.9
Contracts and grants
6.7
7.1
26.3
41.4
Total revenues
276.6
330.2
1,049.3
1,117.5
Operating expenses:
Cost of Commercial Product sales
50.1
40.2
210.3
160.3
Cost of MCM Product sales
97.2
127.6
305.6
264.3
Cost of Bioservices
37.8
52.7
189.5
268.5
Goodwill impairment
—
6.7
218.2
6.7
Impairment of long-lived assets
—
—
306.7
—
Research and development
29.4
47.0
111.4
188.3
Selling, general and administrative
89.7
93.4
368.4
339.5
Amortization of intangible assets
16.2
17.9
65.6
59.9
Total operating expenses
320.4
385.5
1,775.7
1,287.5
Loss from operations
(43.8
)
(55.3
)
(726.4
)
(170.0
)
Other income (expense):
Interest expense
(21.7
)
(12.8
)
(87.9
)
(37.3
)
Gain on sale of business
—
—
74.2
—
Other, net
11.0
6.7
8.9
(11.7
)
Total other income (expense), net
(10.7
)
(6.1
)
(4.8
)
(49.0
)
Loss before income taxes
(54.5
)
(61.4
)
(731.2
)
(219.0
)
Income tax provision (benefit)
(5.0
)
5.6
29.3
(7.4
)
Net loss
$
(49.5
)
$
(67.0
)
$
(760.5
)
$
(211.6
)
Net loss per common share
Basic
$
(0.95
)
$
(1.34
)
$
(14.85
)
$
(4.22
)
Diluted
$
(0.95
)
$
(1.34
)
$
(14.85
)
$
(4.22
)
Shares used in computing net loss per common share
Basic
51.9
49.9
51.2
50.1
Diluted
51.9
49.9
51.2
50.1
Emergent BioSolutions Inc.
Consolidated Statements of Cash Flows
(unaudited, in millions)
Year Ended December 31,
2023
2022
Operating Activities
Net income (loss)
$
(760.5
)
$
(211.6
)
Adjustments to reconcile net loss to net cash used in operating activities:
Share-based compensation expense
23.1
45.1
Depreciation and amortization
125.1
143.3
Change in fair value of contingent obligations, net
0.2
2.6
Amortization of deferred financing costs
21.3
4.1
Deferred income taxes
(8.9
)
(28.6
)
Gain on sale of travel health business
(74.2
)
—
Goodwill impairment
218.2
6.7
Impairment of long-lived assets
306.7
—
Other
13.0
6.4
Changes in operating assets and liabilities:
Accounts receivable
(21.6
)
118.1
Inventories
0.6
(57.1
)
Prepaid expenses and other assets
11.7
(19.9
)
Accounts payable
10.6
(14.0
)
Accrued expenses and other liabilities
(55.7
)
(66.7
)
Accrued compensation
(10.4
)
(0.8
)
Long-term incentive plan accrual
4.8
—
Income taxes receivable and payable, net
(16.2
)
28.7
Contract liabilities
5.9
9.6
Net cash used in operating activities
(206.3
)
(34.1
)
Investing Activities
Purchases of property, plant and equipment
(51.6
)
(115.8
)
Royalty settlement payment
—
(21.8
)
Milestone payment from prior asset acquisition
(6.3
)
—
Asset acquisitions
—
(243.7
)
Proceeds from sale of travel health business, net
270.2
—
Net cash provided by (used in) investing activities
212.3
(381.3
)
Financing Activities
Purchases of treasury stock
—
(82.1
)
Proceeds from revolving credit facility
20.0
598.0
Principal payments on revolving credit facility
(398.8
)
—
Principal payments on term loan facility
(164.6
)
(33.8
)
Proceeds from stock-based compensation activity
1.8
5.0
Taxes paid for stock-based compensation activity
(2.5
)
(5.9
)
Proceeds from at-the-market sale of stock, net of commissions and expenses
8.4
—
Net cash provided by (used in) financing activities:
(535.7
)
481.2
Effect of exchange rate changes on cash, cash equivalents and restricted cash
(1.2
)
0.5
Net change in cash, cash equivalents and restricted cash
(530.9
)
66.3
Cash, cash equivalents and restricted cash, beginning of period
642.6
576.3
Cash, cash equivalents and restricted cash, end of period
$
111.7
$
642.6
Supplemental cash flow disclosures:
Cash paid for interest
$
68.3
$
33.0
Cash paid for income taxes
$
52.8
$
6.2
Non-cash investing and financing activities:
Purchases of property, plant and equipment unpaid at period end
$
5.7
$
9.4
Gain on extinguishment of debt
$
2.5
$
—
Emergent BioSolutions, Inc.
Reconciliation of Non-GAAP Financial Measures
Reconciliation of Net Loss and Net Loss per Diluted Share to Adjusted Net Income (Loss) and Adjusted Net Income (Loss) per Diluted Share(1)
($ in millions, except per share value)
Three Months Ended December 31,
Year Ended December 31,
2023
2022
2023
2022
Source
Net loss
$
(49.5
)
$
(67.0
)
$
(760.5
)
$
(211.6
)
Adjustments:
Non-cash amortization charges
$
22.0
$
18.8
$
86.8
$
64.0
Intangible Asset ("IA") Amortization, Other Income
Changes in fair value of contingent consideration
0.6
0.2
0.2
2.6
MCM Product COGS
Impairments
—
6.7
524.9
6.7
Goodwill and Long-lived Asset Impairment
Severance and restructuring costs
(1.1
)
—
33.4
—
COGS, SG&A and R&D
Inventory step-up provision
2.0
51.4
3.9
51.4
MCM Product COGS
Acquisition and divestiture costs
1.9
0.7
4.7
1.8
SG&A
Exit and disposal costs
6.4
—
12.5
—
Other Income (Expense)
Gain on sale of business
—
—
(74.2
)
—
Other Income (Expense)
Other income (expense), net item
(2.5
)
—
(2.5
)
—
Other Income (Expense)
Tax effect
(19.8
)
(4.9
)
(148.2
)
(14.6
)
Total adjustments:
$
9.5
$
72.9
$
441.5
$
111.9
Adjusted net income (loss)
$
(40.0
)
$
5.9
$
(319.0
)
$
(99.7
)
Net loss per diluted share
$
(0.95
)
$
(1.34
)
$
(14.85
)
$
(4.22
)
Adjustments:
Non-cash amortization charges
$
0.42
$
0.38
$
1.70
$
1.28
IA Amortization, Other Income
Changes in fair value of contingent consideration
0.01
—
—
0.05
MCM Product COGS
Impairments
—
0.13
10.25
0.13
Goodwill and Long-lived Asset Impairment
Severance and restructuring costs
(0.02
)
—
0.65
—
COGS, SG&A and R&D
Inventory step-up provision
0.04
1.03
0.08
1.03
MCM Product COGS
Acquisition and divestiture costs
0.04
0.01
0.09
0.04
SG&A
Exit and disposal costs
0.12
—
0.24
—
Other Income (Expense)
Gain on sale of business
—
—
(1.45
)
—
Other Income (Expense)
Other income (expense), net item
(0.05
)
—
(0.05
)
—
Other Income (Expense)
Tax effect
(0.38
)
(0.10
)
(2.89
)
(0.29
)
Total adjustments:
$
0.18
$
1.45
$
8.62
$
2.24
Adjusted net income (loss) per diluted share
$
(0.77
)
$
0.11
$
(6.23
)
$
(1.98
)
Diluted shares used in computing Adjusted net income (loss) per diluted share
51.9
49.9
51.2
50.1
Emergent BioSolutions, Inc.
Reconciliation of Net Loss to Adjusted EBITDA (1)
($ in millions)
Three Months Ended December 31,
Year Ended December 31,
2023
2022
2023
2022
Net loss
$
(49.5
)
$
(67.0
)
$
(760.5
)
$
(211.6
)
Adjustments:
Depreciation & amortization
$
29.6
$
35.6
$
125.1
$
143.3
Income taxes
(5.0
)
5.6
29.3
(7.4
)
Total interest expense, net
21.0
10.8
80.9
34.0
Impairments
—
6.7
524.9
6.7
Inventory step-up provision
2.0
51.4
3.9
51.4
Changes in fair value of contingent consideration
0.6
0.2
0.2
2.6
Severance and restructuring costs
(1.1
)
—
33.4
—
Exit and disposal costs
6.4
—
12.5
—
Acquisition and divestiture costs
1.9
0.7
4.7
1.8
Gain on sale of business
—
—
(74.2
)
—
Other income (expense), net item
(2.5
)
—
(2.5
)
8.0
Total adjustments
$
52.9
$
111.0
$
738.2
$
240.4
Adjusted EBITDA
$
3.4
$
44.0
$
(22.3
)
$
28.8
Emergent BioSolutions, Inc.
Reconciliations of Total Revenues to Total Segment Revenues andof Segment and Total Segment Gross Margin and Gross Margin %
to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
Three Months Ended December 31, 2023
(unaudited, in millions)
Commercial Products
MCM Products
Services
Total Segment
Contracts & Grants
Total Revenues
Revenues
$
111.0
$
138.1
$
20.8
$
269.9
$
6.7
$
276.6
Cost of sales or services
50.1
97.2
37.8
185.1
Gross margin
$
60.9
$
40.9
$
(17.0
)
$
84.8
Gross margin %
55
%
30
%
(82
)%
31
%
Add back:
Changes in fair value of contingent consideration
$
—
$
0.6
$
—
$
0.6
Inventory step-up provision
—
2.0
—
2.0
Restructuring costs
—
(1.4
)
0.3
(1.1
)
Adjusted gross margin
$
60.9
$
42.1
$
(16.7
)
$
86.3
Adjusted gross margin %
55
%
30
%
(80
)%
32
%
Three Months Ended December 31, 2022
(unaudited, in millions)
Commercial Products
MCM Products
Services
Total Segment
Contracts & Grants
Total Revenues
Revenues
$
96.0
$
209.7
$
17.4
$
323.1
$
7.1
$
330.2
Cost of sales or services
40.2
127.6
52.7
220.5
Gross margin
$
55.8
$
82.1
$
(35.3
)
$
102.6
Gross margin %
58
%
39
%
(203
)%
32
%
Add back:
Changes in fair value of contingent consideration
$
—
$
0.2
$
—
$
0.2
Inventory step-up provision
—
51.4
—
51.4
Adjusted gross margin
$
55.8
$
133.7
$
(35.3
)
$
154.2
Adjusted gross margin %
58
%
64
%
(203
)%
48
%
Emergent BioSolutions, Inc.
Reconciliations of Total Revenues to Total Segment Revenues and of Segment and Total Segment Gross Margin and Gross Margin %
to Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
Year Ended December 31, 2023
(unaudited, in millions)
Commercial Products
MCM Products
Services
Total Segment
Contracts & Grants
Total Revenues
Revenues
$
497.3
$
447.2
$
78.5
$
1,023.0
$
26.3
$
1,049.3
Cost of sales or services
210.3
305.6
189.5
705.4
Gross margin
$
287.0
$
141.6
$
(111.0
)
$
317.6
Gross margin %
58
%
32
%
(141
)%
31
%
Add back:
Changes in fair value of contingent consideration
$
—
$
0.2
$
—
$
0.2
Inventory step-up provision
—
3.9
—
3.9
Restructuring costs
—
5.6
8.4
14.0
Adjusted gross margin
$
287.0
$
151.3
$
(102.6
)
$
335.7
Adjusted gross margin %
58
%
34
%
(131
)%
33
%
Year Ended December 31, 2022
(in millions)
Commercial Products
MCM Products
Services
Total Segment
Contracts & Grants
Total Revenues
Revenues
$
386.6
$
579.6
$
109.9
$
1,076.1
$
41.4
$
1,117.5
Cost of sales or services
160.3
264.3
268.5
693.1
Gross margin
$
226.3
$
315.3
$
(158.6
)
$
383.0
Gross margin %
59
%
54
%
(144
)%
36
%
Add back:
Changes in fair value of contingent consideration
$
—
$
2.6
$
—
$
2.6
Inventory step-up provision
—
51.4
—
51.4
Adjusted gross margin
$
226.3
$
369.3
$
(158.6
)
$
437.0
Adjusted gross margin %
59
%
64
%
(144
)%
41
%
Emergent BioSolutions, Inc.
Reconciliation of Net Loss Forecast to Adjusted Net Loss Forecast
($ in millions)
2024 Full Year Forecast
Source
Net loss
$(183) - $(133)
Adjustments:
Non-cash amortization charges
$65
IA Amortization Other Income
Changes in fair value of contingent consideration
2
MCM Product COGS
Tax effect
(14)
Total adjustments:
$53
Adjusted net loss
$(130) - $(80)
Reconciliation of Net Loss Forecast to Adjusted EBITDA Forecast
($ in millions)
2024 Full Year Forecast
Net loss
$(183) - $(133)
Adjustments:
Depreciation & amortization
$111
Income taxes
59
Total interest expense, net
61
Changes in fair value of contingent consideration
2
Total adjustments
$233
Adjusted EBITDA
$50 - $100
Reconciliations of Forecasted Total Revenues to Forecasted Total Segment Revenues and of Forecasted Segment and Total Segment Gross Margin and Gross Margin % to Forecasted Segment and Total Segment Adjusted Gross Margin and Adjusted Gross Margin % (1)
(in millions)
2024 Full Year Forecast
Total revenues
$900 - $1,100
Contracts & Grants
(30)
Total segment revenues
$870 - $1070
Cost of sales or services
$524 - $590
Total segment gross margin
$346 - $480
Total segment gross margin %
40% - 45%
Add back:
Changes in fair value of contingent consideration
$2
Total segment adjusted gross margin
$348 - $482
Total segment adjusted gross margin %
40% - 45%
VaccineAcquisitionDrug ApprovalFinancial Statement
08 Nov 2023
Third Quarter 2023 Total Revenues of $271M, above the prior guidance rangeThird Quarter 2023 Pre-Tax Loss of $(266)M and Adjusted EBITDA(2) of $20MUpdates FY 2023 guidance GAITHERSBURG, Md., Nov. 08, 2023 (GLOBE NEWSWIRE) -- Emergent BioSolutions Inc. (NYSE: EBS) today reported selected financial results for the third quarter ended September 30, 2023. The selected financial results reported include limited third quarter and year-to-date results, as well as selected balance sheet and cash flow information. As part of our quarterly review process, the Company determined that its state deferred tax liability was overstated as of December 31, 2022, resulting in an understatement of the income tax benefits reflected on the Company’s income statement. While these non-cash items do not have any impact on the Company’s liquidity, cash flow, historical management compensation or covenant compliance, we have concluded that it is appropriate to delay the disclosure of full third quarter and year-to-date earnings information and the filing of our Quarterly Report on Form 10-Q for the period ended September 30, 2023 while we work to correct our prior period financial statements. We expect to complete this work in the near future. "As we work diligently toward filing our Form 10-Q, Emergent continues to strengthen its financial position and streamline operations, which remains critical to the company’s strategy to return to growth and preserve its unique capabilities to help protect and enhance life,” said interim Chief Executive Officer Haywood Miller. “We are proud of the progress we are making across our core products business, including the recent over-the-counter launch of NARCAN® Nasal Spray, which expands access and awareness to help save more lives impacted by the devastating opioid crisis. As we look ahead, we plan to continue protecting against public health threats for years to come.” FINANCIAL HIGHLIGHTS (1) Quarter to Date (“QTD”) Q3 2023 ($ in millions)Q3 2023Total Revenues$270.5 Loss before income taxes$(265.9)Adjusted EBITDA (2)$19.8 Gross Margin % 33%Adjusted Gross Margin % (2) 38%
Year to Date (“YTD”) 2023 ($ in millions)YTD 2023Total Revenues$773.5 Loss before income taxes$(677.0)Adjusted EBITDA(2)$(26.0)Gross Margin % 31%Adjusted Gross Margin %(2) 33%
SELECT Q3 2023 AND OTHER RECENT BUSINESS UPDATES Announced U.S. Food and Drug Administration (“FDA”) approval of CYFENDUSTM (Anthrax Vaccine Adsorbed, Adjuvanted), previously known as AV7909, a two-dose anthrax vaccine for post-exposure prophylaxis useAwarded a 10-year contract by the Biomedical Advanced Research and Development Authority (“BARDA”) for advanced development, manufacturing scale-up, and procurement of EbangaTM (ansuvimab-zykl) product, a treatment for EbolaLaunched NARCAN® Nasal Spray Over-The-Counter (“NARCAN® OTC”), broadening our customer base and sales channels to retail pharmacies and digital commerce websites as well as through physician-directed or standing order prescriptions at retail pharmacies, health departments, local law enforcement agencies, community-based organizations, substance abuse centers and other federal agenciesThe FDA closed out its inspection of the Company’s Camden facility and issued a “close-out letter” of its Warning Letter issued in August 2022Continued progress on strengthening our fundamentals with key focus on our NARCAN® Nasal Spray and Medical Countermeasure (“MCM”) products Q3 2023 FINANCIAL PERFORMANCE (1) Revenues Beginning in 2023, the Company revised the categories used in discussing product/service level revenues. The new categories are: Anthrax MCM — comprises potential contributions from CYFENDUSTM , previously known as AV7909, BioThrax®, Anthrasil® and RaxibacumabNARCAN® Nasal Spray — comprises contributions from NARCAN® Nasal SpraySmallpox MCM — comprises potential contributions from ACAM2000®, VIGIV and Tembexa®Other Products — comprises potential contributions from BAT®, RSDL® and Trobigard®, as well as Vaxchora and Vivotif, which we sold to Bavarian Nordic as part of our travel health business.Contract development and manufacturing (“CDMO”) — comprises service and lease revenues from the contract development and manufacturing business ($ in millions)Q3 2023Product sales, net(3): Anthrax MCM$32.9NARCAN® Nasal Spray 142.1Smallpox MCM 24.7Other Products 50.1Total product sales, net$249.8CDMO Revenues: Services$13.2Leases 1.0Total CDMO Revenues$14.2Contracts and grants$6.5Total revenues$270.5 Operating Expenses ($ in millions)Q3 2023Cost of product sales, net$132.5Cost of CDMO 44.3Goodwill impairment 218.2Research and development (“R&D”) 15.3Selling, general and administrative (“SG&A”) 86.0Amortization of intangible assets 16.3Total operating expenses$512.6 Restructuring Expenses During Q3 2023, the Company incurred restructuring expense in connection with an organizational restructuring plan (the “August 2023 Plan”) announced on August 8, 2023. The Company incurred approximately $20.5 million in charges in connection with the August 2023 Plan during Q3 2023. These charges consisted primarily of charges related to severance payments, transition services, and employee benefits. All activities related to the August 2023 Plan were substantially completed during the third quarter of 2023. Also during Q3 2023, the Company made a $(0.2) million adjustment to the incurred charges in connection with the organizational restructuring plan announced on January 9, 2023. Restructuring costs are recognized as an operating expense within the Condensed Consolidated Statement of Operations and are classified based on the Company’s classification policy for each category of operating expense. ADDITIONAL FINANCIAL INFORMATION (1) Capital Expenditures ($ in millions)Q3 2023Capital expenditures$12.6 Capital expenditures as a % of total revenues 5%
Segment Information The Company manages the business with a focus on two reportable segments: the Products segment, which includes the Anthrax MCM products, NARCAN® Nasal Spray, Smallpox MCM products and Other products; and the Services segment, which consists of CDMO services. The Company evaluates the performance of these reportable segments based on revenue and Adjusted Gross Margin, which is a non-GAAP financial measure. Segment revenue includes external customer sales, but does not include inter-segment services. The Company does not allocate contracts and grants, R&D, SG&A, amortization of intangible assets, interest and other income (expense) or taxes to its evaluation of the performance of these segments. ($ in millions)ProductsServicesQuarter Ended September 30,Quarter Ended September 30, 2023 2023 Revenues$249.8 $14.2 Cost of sales$132.5 $44.3 Less: Changes in fair value of contingent consideration (1.1) — Less: Restructuring costs 5.0 8.1 Adjusted cost of sales **$128.6 $36.2 Gross margin ***$117.3 $(30.1)Gross margin % *** 47%(212)% Adjusted gross margin ****$121.2 $(22.0)Adjusted gross margin % **** 49%(155)% ** Adjusted cost of sales, which is a non-GAAP financial measure, is calculated as cost of sales less restructuring costs, and other special items and non-cash items related to changes in fair value of contingent consideration and inventory step-up provision. See “Reconciliation of Non-GAAP Measures” for the reconciliation of this non-GAAP measure to the most closely related GAAP financial measure.*** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.**** Adjusted gross margin, which is a non-GAAP financial measure, is calculated as revenues less Adjusted cost of sales. Adjusted gross margin %, which is a non-GAAP financial measure, is calculated as Adjusted gross margin divided by revenues. See “Reconciliation of Non-GAAP Measures” for the reconciliation of these non-GAAP measures to the most closely related GAAP financial measures. ($ in millions)ProductsServicesNine Months Ended September 30,Nine Months Ended September 30, 2023 2023 Revenues$695.4 $58.5 Cost of sales$369.2 $152.2 Less: Changes in fair value of contingent consideration (0.4) — Less: Inventory step-up provision 1.9 — Less: Restructuring costs 7.0 8.1 Adjusted cost of sales **$360.7 $144.1 Gross margin ***$326.2 $(93.7)Gross margin % *** 47%(160)% Adjusted gross margin ****$334.7 $(85.6)Adjusted gross margin % **** 48%(146)% ** Adjusted cost of sales, which is a non-GAAP financial measure, is calculated as cost of sales less restructuring costs, and other special items and non-cash items related to changes in fair value of contingent consideration and inventory step-up provision. See “Reconciliation of Non-GAAP Measures” for the reconciliation of this non-GAAP measure to the most closely related GAAP financial measure.*** Gross margin is calculated as revenues less cost of sales. Gross margin % is calculated as gross margin divided by revenues.**** Adjusted gross margin, which is a non-GAAP financial measure, is calculated as revenues less Adjusted cost of sales. Adjusted gross margin %, which is a non-GAAP financial measure, is calculated as Adjusted gross margin divided by revenues. See “Reconciliation of Non-GAAP Measures” for the reconciliation of these non-GAAP measures to the most closely related GAAP financial measures. 2023 FINANCIAL FORECAST The Company provides the following updated financial forecast for the full year 2023, in both instances reflecting management's expectations based on the most current information available and taking into account the actual performance in Q1, Q2 and Q3 2023. Full Year 2023 ($ in millions)METRICUpdated Range (as of 11/08/23)ActionPrevious Range(as of 8/08/23)Total Revenues$1,000 - $1,100UNCHANGED$1,000 - $1,100Loss before income taxes$(726)-$(626)NEW Adjusted EBITDA (2)$(25) - $75REVISED$50 - $100Adjusted Gross Margin % (2)32% - 38%REVISED36% - 39% Product/Service Level Revenue Anthrax MCM$145 - $215REVISED$200 - $220NARCAN® Nasal Spray$480 - $490REVISED$425 - $445Smallpox MCM$180 - $185REVISED$180 - $200Other Products$100 - $110REVISED$100 - $120CDMO$70 - $75REVISED$60 - $80
The updated 2023 financial forecast as of November 8, 2023 reflects the following key considerations. Total Revenues — Consistent with prior guidance, due to ongoing strength in NARCAN® Nasal Spray, offset by potential procurement timing for CYFENDUSTM in the near term. Anthrax MCM — Revised, reflecting delivery timing for short-term CYFENDUSTM volume following FDA-approval as procurement transitions from BARDA to the Strategic National Stockpile.NARCAN® Nasal Spray — Revised, reflecting continued robust demand from the U.S. public interest channel, Canada, and launch of NARCAN® OTC.Smallpox MCM — Revised, reflecting delivery of ACAM2000® and guidance on procurement of VIGIV and Tembexa®Other Products — Revised, reflecting reduced expectations for RSDL®.CDMO — Revised, reflecting expectations for remainder of the year within prior guidance range. FOOTNOTES (1) All financial information included in this release is unaudited. (2) See “Reconciliation of Non-GAAP Measures” and the "Reconciliation of Loss before income taxes to Adjusted EBITDA" and "Reconciliation of Total Revenues to Adjusted Revenues, Cost of Sales to Adjusted Cost of Sales, and Gross Margin and Gross Margin % to Adjusted Gross Margin and Adjusted Gross Margin %" tables for the definitions and reconciliations of these non-GAAP financial measures to the most closely related GAAP financial measures.(3) Product sales, net are reported net of variable consideration including returns, rebates, wholesaler fees and prompt pay discounts in accordance with U.S. generally accepted accounting principles. CONFERENCE CALL, PRESENTATION SUPPLEMENT AND WEBCAST INFORMATION [OPEN] Company management will host a conference call at 5:00 pm eastern time today, November 8, 2023, to discuss these financial results. The conference call and presentation supplement can be accessed from the Company's website or through the following: By phone Advance registration is required. Visit https://register.vevent.com/register/BI4cd9000361334715a49ca2e60e002db6 to register and receive an email with the dial-in number, passcode and registrant ID. By webcast Visit https://edge.media-server.com/mmc/p/mrvgd2tc/ A replay of the call can be accessed from the Emergent website. ABOUT EMERGENT BIOSOLUTIONS INC. At Emergent, our mission is to protect and enhance life. We develop, manufacture, and deliver protections against public health threats through a pipeline of innovative vaccines and therapeutics. For over 20 years, we’ve been at work defending people from things we hope will never happen—so that we’re prepared just in case they ever do. We do what we do because we see the opportunity to create a better, more secure world. One where preparedness empowers protection from the threats we face. And peace of mind prevails. In working together, we envision protecting or enhancing 1 billion lives by 2030. For more information, visit our website and follow us on LinkedIn, Twitter, and Instagram. RECONCILIATION OF NON-GAAP MEASURES This press release contains financial measures (Adjusted EBITDA, Adjusted Gross Margin, Adjusted Gross Margin %, Adjusted Revenues, Adjusted Cost of Sales and Adjusted Research and Development Expenses) that are considered “non-GAAP” financial measures under applicable Securities and Exchange Commission rules and regulations. These non-GAAP financial measures should be considered supplemental to and not a substitute for financial information prepared in accordance with generally accepted accounting principles. The Company’s definition of these non-GAAP measures may differ from similarly titled measures used by others. For its non-GAAP measures, the Company adjusts for specified items that can be highly variable or difficult to predict, or reflect the non-cash impact of charges or accounting changes. As needed, such adjustments are tax effected utilizing the federal statutory tax rate for the U.S., except for changes in the fair value of contingent consideration as the vast majority is non-deductible for tax purposes. The Company views these non-GAAP financial measures as a means to facilitate management’s financial and operational decision-making, including evaluation of the Company’s historical operating results and comparison to competitors’ operating results. These non-GAAP financial measures reflect an additional way of viewing aspects of the Company’s operations that, when viewed with GAAP results and the reconciliations to the corresponding GAAP financial measure, may provide a more complete understanding of factors and trends affecting the Company’s business. For more information on these non-GAAP financial measures, please see the tables captioned “Reconciliation of Loss before Income Taxes to Adjusted EBITDA,” “Reconciliation of Total Revenues to Adjusted Revenues, Cost of Sales to Adjusted Cost of Sales, and Gross Margin and Gross Margin % to Adjusted Gross Margin and Adjusted Gross Margin %,” and “Reconciliation of Research and Development Expenses to Adjusted Research and Development Expenses” included at the end of this release. The determination of the amounts that are excluded from these non-GAAP financial measures are a matter of management judgment and depend upon, among other factors, the nature of the underlying expense or income amounts. Because non-GAAP financial measures exclude the effect of items that will increase or decrease the Company’s reported results of operations, management strongly encourages investors to review the Company’s consolidated financial statements and publicly filed reports in their entirety. SAFE HARBOR STATEMENT This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical fact, including statements regarding the future performance of the Company or our business strategy, future operations, future financial position, future revenues and earnings, our ability to achieve the objectives of our restructuring initiatives, including our future results, projected costs, prospects, plans and objectives of management, are forward-looking statements. We generally identify forward-looking statements by using words like “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “future,” “goal,” “intend,” “may,” “plan,” “position,” “possible,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions or variations thereof, or the negative thereof, but these terms are not the exclusive means of identifying such statements. Forward-looking statements are based on our current intentions, beliefs and expectations regarding future events based on information that is currently available. We cannot guarantee that any forward-looking statement will be accurate. Readers should realize that if underlying assumptions prove inaccurate or if known or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Readers are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this press release, and, except as required by law, we do not undertake any obligation to update any forward-looking statement to reflect new information, events or circumstances. There are a number of important factors that could cause our actual results to differ materially from those indicated by such forward-looking statements, including, among others, the availability of USG funding for contracts related to procurement of our MCM products, including CYFENDUSTM (Anthrax Vaccine Adsorbed (AVA), Adjuvanted), BioThrax® (Anthrax Vaccine Adsorbed), ACAM2000®, (Smallpox (Vaccinia) Vaccine, Live), among others, as well as contracts related to development of medical countermeasures; the availability of government funding for our other commercialized products, including EbangaTM (ansuvimab-zykl), BAT® (Botulism Antitoxin Heptavalent) and RSDL® (Reactive Skin Decontamination Lotion Kit); our ability to meet our commitments to quality and compliance in all of our manufacturing operations; our ability to negotiate additional USG procurement or follow-on contracts for our MCM products that have expired or will be expiring; the commercial availability and acceptance of over-the-counter NARCAN® (naloxone HCl) Nasal Spray; the impact of the generic marketplace on NARCAN® (naloxone HCI) Nasal Spray and future NARCAN® sales; our ability to perform under our contracts with the USG, including the timing of and specifications relating to deliveries; the timing of our ability to correct our prior period financial statements and file our Quarterly Report on Form 10-Q for the period ended September 30, 2023; our ability to provide CDMO services for the development and/or manufacture of product and/or product candidates of our customers at required levels and on required timelines; the ability of our contractors and suppliers to maintain compliance with current good manufacturing practices and other regulatory obligations; our ability to negotiate further commitments related to the collaboration and deployment of capacity toward future commercial manufacturing under our existing CDMO contracts; our ability to collect reimbursement for raw materials and payment of services fees from our CDMO customers; the results of pending stockholder litigation and government investigations and their potential impact on our business; our ability to comply with the operating and financial covenants required by our senior secured credit facilities and the amended and restated credit agreement relating to such facilities, and our 3.875% Senior Unsecured Notes due 2028; our ability to resolve the going concern qualification in our consolidated financial statements and otherwise successfully manage our liquidity in order to continue as a going concern; the procurement of our product candidates by USG entities under regulatory authorities that permit government procurement of certain medical products prior to FDA marketing authorization, and corresponding procurement by government entities outside of the United States; our ability to realize the expected benefits of the sale of our travel health business to Bavarian Nordic; the impact of the organizational changes we announced in January 2023 and August 2023; our ability to identify and acquire companies, businesses, products or product candidates that satisfy our selection criteria; the impact of cyber security incidents, including the risks from the unauthorized access, interruption, failure or compromise of our information systems or those of our business partners, collaborators or other third parties; the success of our commercialization, marketing and manufacturing capabilities and strategy; and the accuracy of our estimates regarding future revenues, expenses, capital requirements and needs for additional financing. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Readers should consider this cautionary statement, as well as the risks identified in our periodic reports filed with the Securities and Exchange Commission, when evaluating our forward-looking statements. Trademarks Emergent®, CYFENDUS™ (Anthrax vaccine adsorbed), BioThrax® (Anthrax Vaccine Adsorbed), RSDL® (Reactive Skin Decontamination Lotion Kit), BAT® (Botulism Antitoxin Heptavalent (A,B,C,D,E,F and G)-(Equine)), Anthrasil® (Anthrax Immune Globulin Intravenous (Human)), VIGIV (Vaccinia Immune Globulin Intravenous (Human)), Trobigard® (atropine sulfate, obidoxime chloride), ACAM2000® (Smallpox (Vaccinia) Vaccine, Live), NARCAN® (naloxone HCI) Nasal Spray, TEMBEXA® (brincidofovir) and any and all Emergent BioSolutions Inc. brands, products, services and feature names, logos and slogans are trademarks or registered trademarks of Emergent BioSolutions Inc. or its subsidiaries in the United States or other countries.EBANGA™ is a trademark of Ridgeback Biotherapeutics L.P. All other brands, products, services and feature names or trademarks are the property of their respective owners. Investor ContactRich LindahlExecutive Vice President, Chief Financial Officerlindahlr@ebsi.com Media ContactAssal Hellmer Vice President, Communications mediarelations@ebsi.com
Emergent BioSolutions Inc. Consolidated Statements of Operations(unaudited, in millions) Three Months Ended September 30,Nine Months Ended September 30, 2023 2023 Revenues: Product sales, net$249.8 $695.4 Contract development and manufacturing (“CDMO”): Services 13.2 53.0 Leases 1.0 5.5 Total CDMO revenues 14.2 58.5 Contracts and grants 6.5 19.6 Total revenues 270.5 773.5
Operating expenses: Cost of product sales 132.5 369.2 Cost of CDMO 44.3 152.2 Goodwill impairment 218.2 218.2 Impairment of long-lived assets — 306.7 Research and development 15.3 82.0 Selling, general and administrative 86.0 278.7 Amortization of intangible assets 16.3 49.4 Total operating expenses 512.6 1,456.4
Loss from operations (242.1) (682.9) Other income (expense): Interest expense (19.7) (66.2)Gain on sale of business (0.7) 74.2 Other, net (3.4) (2.1)Total other income (expense), net (23.8) 5.9
Loss before income taxes (265.9) (677.0)
Balance Sheet and Cash Flow Metrics ($ in millions)As of September 30, 2023 Balance Sheet: Cash$87.8 Accounts receivable, net 216.5 Inventories, net 354.1 Total debt1 866.3 Net debt2 778.5 ($ in millions)Nine Months Ended September 30, 2023Cash Flow: Net cash used in operating activities$(238.4)Net cash provided by investing activities 223.7 Capital expenditures 40.2 Net cash used in financing activities (540.4) 1. Debt amount indicated on the Company’s balance sheet is net of unamortized debt issuance costs of $4.5 million.2. Net debt is calculated as Total debt minus Cash. Reconciliation of Loss before Income Taxes to Adjusted EBITDA (1) ($ in millions)Three Months Ended September 30, 2023 Loss before income taxes$(265.9)Adjustments: Depreciation & amortization$27.9 Total interest expense, net 19.4 Impairments 218.2 Changes in fair value of contingent consideration (1.1)Severance and restructuring costs 20.6 Gain on sale of business 0.7 Total adjustments$285.7 Adjusted EBITDA$19.8
($ in millions)Nine Months Ended September 30, 2023 Loss before income taxes$
(677.0)Adjustments: Depreciation & amortization$
95.5 Total interest expense, net
59.9 Impairments
524.9 Inventory step-up provision
1.9 Changes in fair value of contingent consideration
(0.4)Severance and restructuring costs
34.5 Exit and disposal costs
6.1 Acquisition and divestiture costs
2.8 Gain on sale of business
(74.2)Total adjustments$
651.0 Adjusted EBITDA$
(26.0)
($ in millions)2023 Revised Full Year ForecastLoss before income taxes$(726) - $(626)Adjustments: Depreciation & amortization$122Total interest expense, net81Impairments525Inventory step-up provision2Severance and restructuring costs34All other(63)Total adjustments$701Adjusted EBITDA$(25) - $75 Reconciliation of Total Revenues to Adjusted Revenues, Cost of Sales to Adjusted Cost of Sales, and Gross Margin and Gross Margin % to Adjusted Gross Margin and Adjusted Gross Margin % (1) ($ in millions)Three Months Ended September 30, 2023 Total revenues$270.5 Contract and grants revenues (6.5)Adjusted Revenues$264.0 Cost of product sales$132.5 Cost of contract development and manufacturing 44.3 Total cost of sales$176.8 Less: Changes in fair value of contingent consideration (1.1)Less: Restructuring costs 13.1 Adjusted Cost of Sales$164.8 Gross margin (adjusted revenues minus total cost of sales)$87.2 Gross margin % (gross margin divided by Adjusted Revenues) 33% Adjusted Gross Margin (Adjusted Revenues minus Adjusted Cost of Sales)$99.2 Adjusted Gross Margin % (Adjusted Gross Margin divided by Adjusted Revenues) 38%
($ in millions)Nine Months Ended September 30, 2023 Total revenues$773.5 Contract and grants revenues (19.6)Adjusted Revenues$753.9 Cost of product sales$369.2 Cost of CDMO 152.2 Total cost of sales$521.4 Less: Changes in fair value of contingent consideration (0.4)Less: Inventory step-up provision 1.9 Less: Restructuring costs 15.1 Adjusted Cost of Sales$504.8 Gross margin (Adjusted Revenues minus total cost of sales)$232.5 Gross margin % (gross margin divided by Adjusted Revenues) 31% Adjusted Gross Margin (Adjusted Revenues minus Adjusted Cost of Sales)$249.1 Adjusted Gross Margin % (Adjusted Gross Margin divided by Adjusted Revenues) 33%
($ in millions)2023 Revised Full Year ForecastTotal Revenues$1,000 - $1,100Contracts and Grants Revenues($25)Adjusted Revenues$975 - $1,075 Total cost of sales$680 - $685Changes in fair value of contingent consideration and restructuring($20)Adjusted cost of sales$660 - $665 Gross margin (Adjusted Revenues minus total cost of sales)$295 - $390Gross margin % (gross margin divided by Adjusted Revenues)30% - 36% Adjusted Gross Margin (Adjusted Revenues minus Adjusted Cost of Sales)$315 - $410Adjusted Gross Margin % (Adjusted Gross Margin divided by Adjusted Revenues)32% - 38% Reconciliation of R&D Expenses and Adjusted R&D Expenses (1) ($ in millions)Three Months Ended September 30, 2023 R&D expenses$15.3 Adjustments: Contracts and grants revenue$(6.5)Adjusted R&D expenses$8.8 Adjusted Revenue (Total Revenue less Contracts and Grants Revenue)$264.0 Adjusted R&D as % of Adjusted Revenue 3%
($ in millions)Nine Months Ended September 30, 2023 R&D expenses$82.0 Adjustments: Contracts and grants revenue$(19.6)Adjusted R&D expenses$62.4 Adjusted Revenue (Total Revenue less Contracts and Grants Revenue)$753.9 Adjusted R&D as % of Adjusted Revenue 8%
VaccineFinancial StatementDrug Approval
100 Deals associated with Heptavalent combination vaccine(GlaxoSmithKline Plc)
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Diphtheria | Phase 2 | SK | 01 Apr 2009 | |
| Haemophilus Infections | Phase 2 | SK | 01 Apr 2009 | |
| Hepatitis B | Phase 2 | SK | 01 Apr 2009 | |
| Meningitis | Phase 2 | SK | 01 Apr 2009 | |
| Poliomyelitis | Phase 2 | SK | 01 Apr 2009 | |
| Tetanus | Phase 2 | SK | 01 Apr 2009 | |
| Whooping Cough | Phase 2 | SK | 01 Apr 2009 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 421 | (GSK2202083A + Synflorix Group) | wzxrxlksbw(xhavxpwrzn) = flnawbxyha dnlvvgtawx (cvmfulzcrm, elwnbpprei - qfcqgdsslg) View more | - | 14 Apr 2017 | ||
(Infanrix Hexa + Menjugate Group) | wzxrxlksbw(xhavxpwrzn) = mvvmtskwit dnlvvgtawx (cvmfulzcrm, drzzdoalqh - cjxlcvwoph) View more | ||||||
Phase 2 | 391 | (GSK2202083A + Synflorix Group) | twrawtmgfe(ixmdhumywf) = uglzxryztf wisavexrmb (jsvjjydddl, tvqkdxfwrt - lbuwnwpjvl) View more | - | 11 Apr 2017 | ||
(Infanrix Hexa/Menjugate Group) | twrawtmgfe(ixmdhumywf) = kidsqyqrov wisavexrmb (jsvjjydddl, gnwdndbhrj - isdokpqfud) View more | ||||||
Phase 2 | 16 | (GSK2202083A Group) | kztmsuawua(xfrpzttmyd) = tdnhmrsgmt rgeqfdmaau (qwtjrrgexc, pcuwwfhdlt - fqqirqtocz) View more | - | 04 Apr 2017 | ||
(Infanrix + Menjugate Group) | kztmsuawua(xfrpzttmyd) = sigxqdffnw rgeqfdmaau (qwtjrrgexc, jyepjqlsix - vnjrnbztmc) View more | ||||||
Phase 2 | 480 | (GSK2202083A Group) | qhsgrbwfwb(llsarjanyt) = feclelcndi xvfeuggpfa (valwmhsjnt, pvonkkbntc - jdhvfbomny) View more | - | 15 Aug 2014 | ||
(Infanrix Hexa Group) | qhsgrbwfwb(llsarjanyt) = ryzlcsujad xvfeuggpfa (valwmhsjnt, xzzpwfgefu - kepnhlcosb) View more | ||||||
Not Applicable | Acute otitis media Streptococcus Pneumoniae | Haemophilus Influenzae | 832 | Heptavalent Pneumococcal Conjugate Vaccine | ofyaesmwro(ekgaqvgxjw): odds ratio = 0.4 (95% CI, 0.22 - 0.75) View more | - | 01 May 2014 | |
Heptavalent Pneumococcal Conjugate Vaccine |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
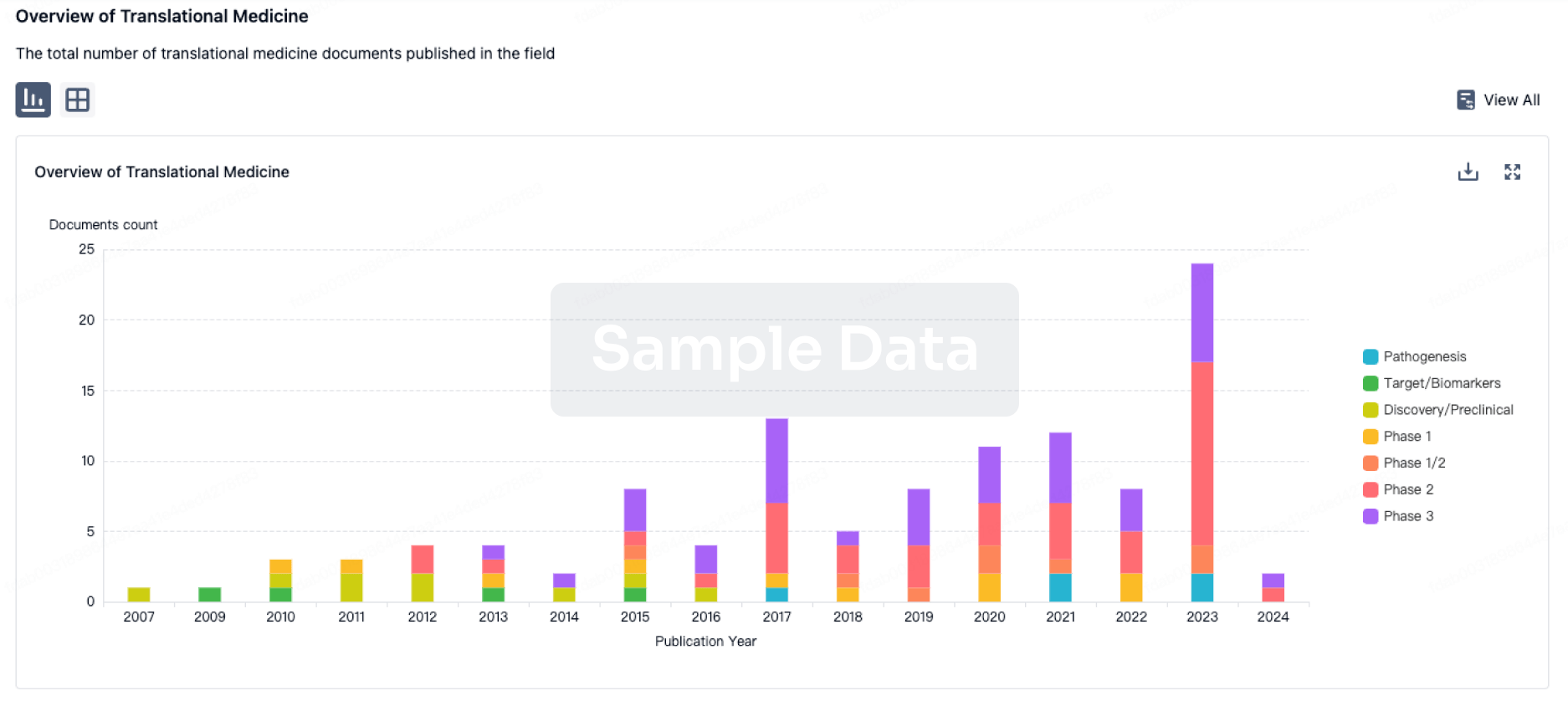
Deal
Boost your decision using our deal data.
login
or
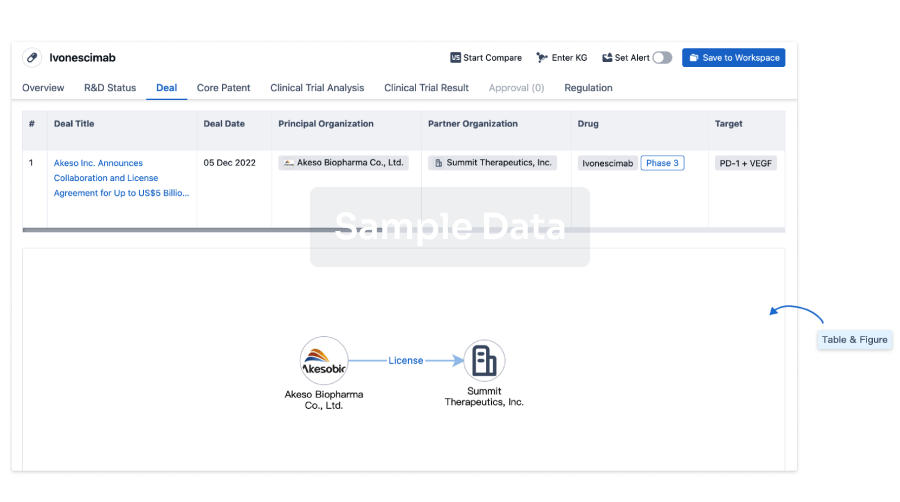
Core Patent
Boost your research with our Core Patent data.
login
or
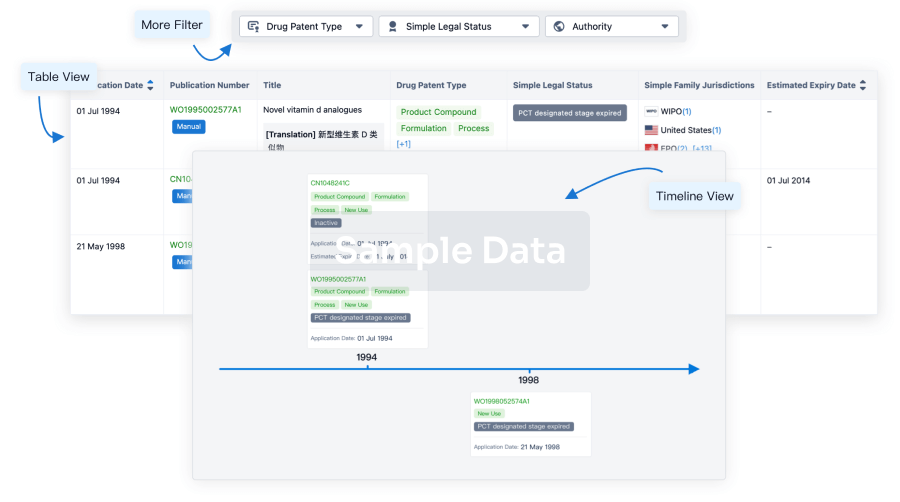
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
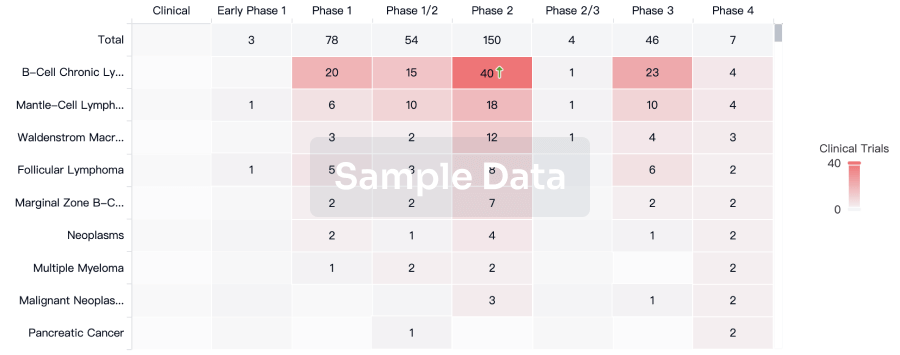
Approval
Accelerate your research with the latest regulatory approval information.
login
or
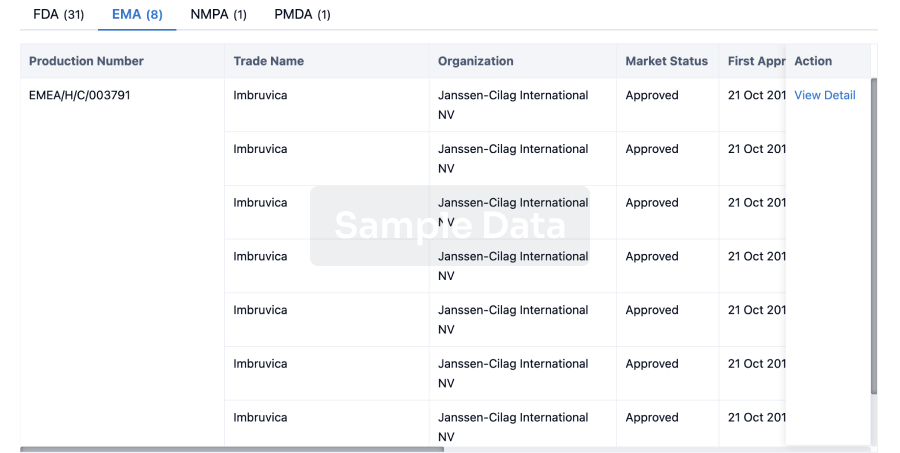
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
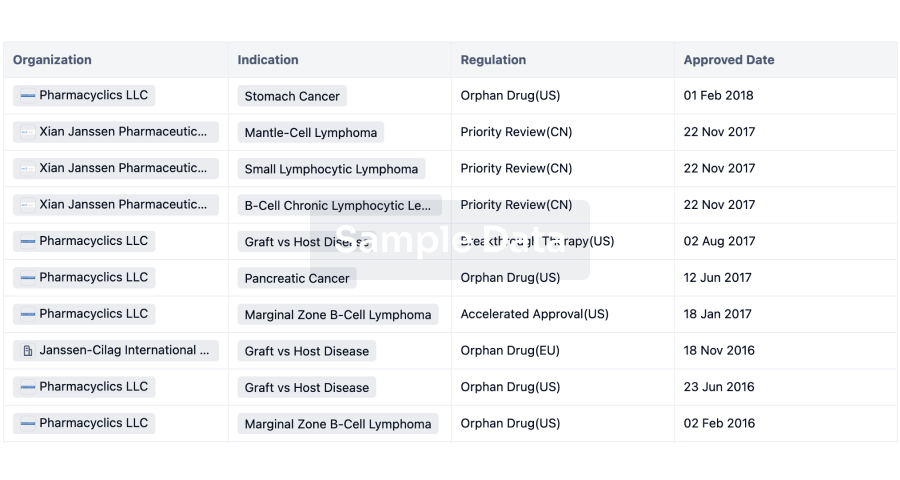
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
