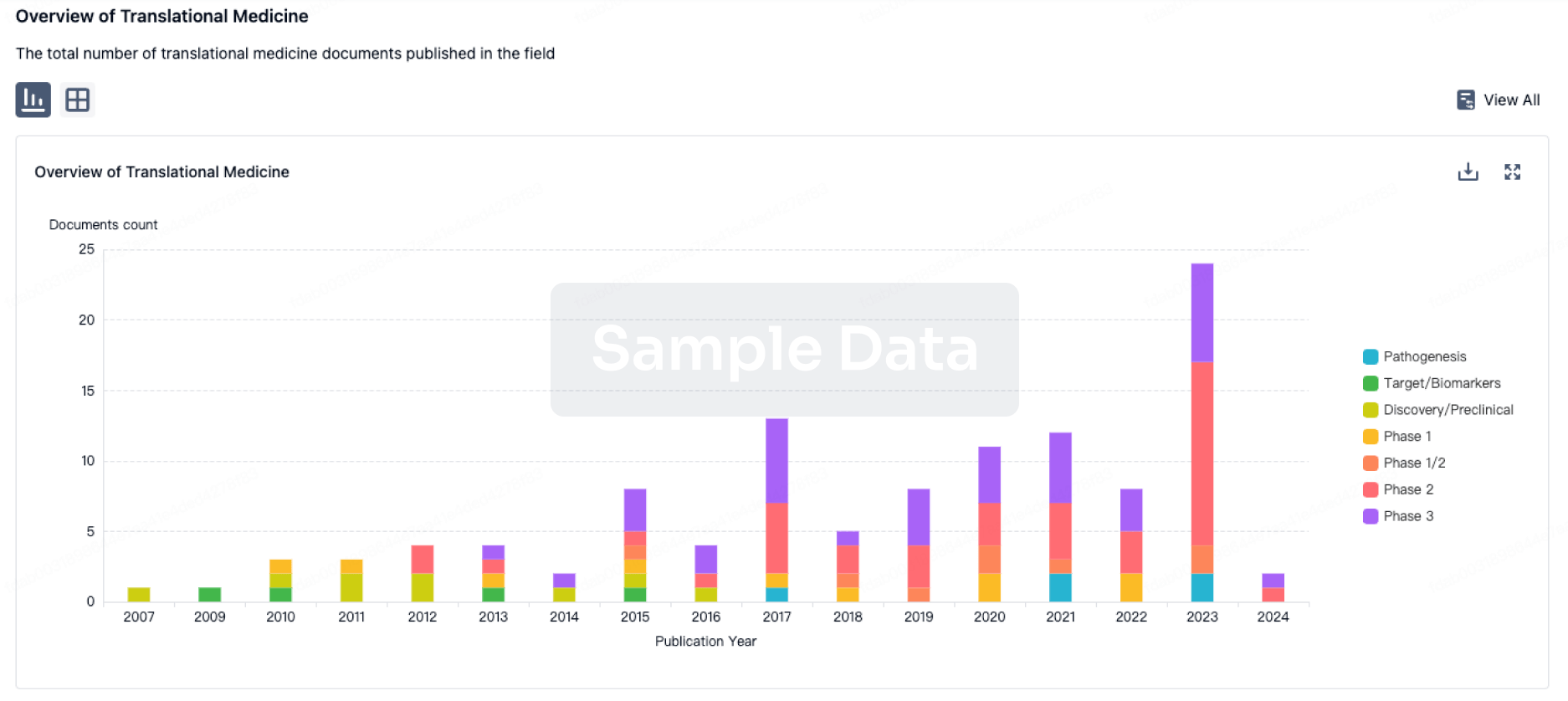
Integration of AL102 and IM-1021 completed
Topline data for Phase 3 RINGSIDE trial of AL102 expected in second half of 2025
IM-1021 and IM-3050 IND submissions expected in first quarter of 2025
BOTHELL, Wash.--(BUSINESS WIRE)-- Immunome, Inc. (Nasdaq: IMNM), a biotechnology company focused on developing first-in-class and best-in-class targeted cancer therapies, today announced financial results for the first quarter ended March 31, 2024, and provided a business update.
“Immunome continues to build momentum. We have completed the integration of AL102 and are executing activities necessary for regulatory submissions,” said Clay B. Siegall, Ph.D., President and Chief Executive Officer. “We have also completed the integration of IM-1021 and are advancing that program and IM-3050 towards clinical trials.”
Dr. Siegall continued, “In addition, we are working internally to design and develop additional next-generation ADC technology. Those efforts are complemented by our ongoing business development efforts, which seek to identify and procure high-quality technologies and clinical programs. Both our internal and external efforts are focused on expanding Immunome’s pipeline with additional programs that have first-in-class or best-in-class potential and can improve the lives of cancer patients.”
Pipeline Highlights
The purchase of AL101 and AL102 from Ayala Pharmaceuticals closed on March 25, 2024. Full enrollment for the Phase 3 RINGSIDE Part B study of AL102 for the treatment of desmoid tumors was completed in February 2024. Immunome expects to report topline data for RINGSIDE Part B in the second half of 2025. In parallel, Immunome continues to perform additional manufacturing and pharmacology work required to support a new drug application filing for AL102.
Immunome anticipates submitting INDs for two preclinical candidates in the first quarter of 2025: IM-1021, a ROR1 ADC, and IM-3050, a FAP-targeted RLT.
First Quarter 2024 Financial Results
As of March 31, 2024, cash, cash equivalents and marketable securities totaled $309.7 million. Immunome’s current cash runway is expected to extend into 2026.
Research and development expenses for the quarter ended March 31, 2024 were $15.4 million, including stock-based compensation costs of $0.4 million.
In-process research and development expenses for the quarter ended March 31, 2024 were $112.0 million. These expenses were related to Immunome’s acquisition of AL101 and AL102 from Ayala Pharmaceuticals, Inc. and the exclusive license of IM-1021 and related ADC platform technology from Zentalis Pharmaceuticals, Inc., both of which closed in the quarter ended March 31, 2024.
General and administrative expenses for the quarter ended March 31, 2024 were $6.0 million, including stock-based compensation expense of $1.8 million.
Immunome reported a net loss of $129.5 million, or basic and diluted net loss per share attributable to common stockholders of $2.51, for the quarter ended March 31, 2024.
About Immunome, Inc.
Immunome is a biotechnology company dedicated to developing first-in-class and best-in-class targeted cancer therapies. Our portfolio pursues each target with a modality appropriate to its biology, including small molecules, ADCs, and RLTs. We believe that pursuing underexplored targets with appropriate drug modalities leads to transformative therapies. Our proprietary memory B cell hybridoma technology allows for the rapid screening and functional characterization of novel antibodies and targets.
For more information, visit or follow us on Twitter and LinkedIn.
Cautionary Statement Regarding Forward-Looking Statements
Certain statements contained in this communication regarding matters that are not historical facts, are forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and the Private Securities Litigation Reform Act of 1995 (the “PSLRA”). We use words such as “may,” “could,” “potential,” “will,” “plan,” “believe,” “expect,” and similar expressions to identify these forward-looking statements that are intended to be covered by the safe-harbor provisions of the PSLRA. These forward-looking statements include, but are not limited to, Immunome’s expectation to report topline data for the ongoing Phase 3 RINGSIDE Part B trial of AL102 in the second half of 2025; Immunome’s expected timeline for submitting INDs for IM-3050 and IM-1021 in the first quarter of 2025; Immunome’s expected cash runway; Immunome’s ability to expand its pipeline through both internal and external efforts; and other statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future. No forward-looking statement can be guaranteed, and actual results may differ materially from those projected. Such forward-looking statements are based on Immunome’s expectations and involve risks and uncertainties; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including, but not limited to, the risk that Immunome will not be able to realize the benefits of its strategic transactions; Immunome’s ability to grow and successfully execute on its business plan, including the development and commercialization of its pipeline and integration of newly acquired assets; changes in the applicable laws or regulations; the possibility that Immunome may be adversely affected by other economic, business, and/or competitive factors; the risk that regulatory approvals for Immunome’s programs and product candidates are not obtained, are delayed or are subject to unanticipated conditions; the risk that pre-clinical data may not be predictive of clinical data; the risk that interim results of a clinical trial do not necessarily predict final results; potential delays in the commencement, enrollment and completion of clinical trials and the reporting of data therefrom; the risk that Immunome’s product and development candidates fail to achieve their intended endpoints; the complexity of numerous regulatory and legal requirements that Immunome needs to comply with to operate its business; the reliance on Immunome’s management; the prior experience and successes of the Immunome’s management team not being indicative of any future success; uncertainties related to Immunome’s capital requirements and Immunome’s expected cash runway; the failure to obtain, adequately protect, maintain or enforce Immunome’s intellectual property rights; and other risks and uncertainties indicated from time to time described in Immunome’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 26, 2024, in ARS Pharma’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2024, being filed with the SEC today, and in Immunome’s other filings with the SEC. Immunome cautions that the foregoing list of factors is not exclusive and not to place undue reliance upon any forward-looking statements which speak only as of the date made. Moreover, Immunome operates in a very competitive and rapidly changing environment. New risks emerge from time to time. Except as required by law, Immunome does not undertake any obligation to update publicly any forward-looking statements for any reason after the date of this press release to conform these statements to actual results or to changes in their expectations.
Immunome, Inc.
Condensed Consolidated Balance Sheets
(Unaudited; In thousands)
March 31, 2024
December 31, 2023
Assets
Current assets:
Cash and cash equivalents
$
269,723
$
98,679
Marketable securities
39,983
39,463
Prepaid expenses and other current assets
3,620
6,561
Total current assets
313,326
144,703
Property and equipment, net
4,302
2,073
Operating right-of-use assets
1,458
1,564
Restricted cash
100
100
Other long-term assets
568
100
Total assets
$
319,754
$
148,540
Liabilities and stockholders’ equity
Current liabilities:
Accounts payable
$
7,179
$
3,311
Accrued expenses and other current liabilities
10,844
8,025
Deferred revenue, current
12,745
10,493
Total current liabilities
30,768
21,829
Deferred revenue, non-current
2,208
5,489
Operating lease liabilities, net of current portion
1,206
1,340
Total liabilities
34,182
28,658
Stockholders’ equity:
Preferred stock
—
—
Common stock
6
4
Additional paid-in capital
637,861
342,663
Accumulated other comprehensive income
4
22
Accumulated deficit
(352,299
)
(222,807
)
Total stockholders’ equity
285,572
119,882
Total liabilities and stockholders’ equity
$
319,754
$
148,540
Immunome, Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited; In thousands, except share and per share amounts)
Three Months Ended March 31,
2024
2023
Collaboration revenue
$
1,029
$
2,364
Operating expenses:
In-process research and development
111,954
—
Research and development(1)
15,369
3,913
General and administrative(1)
6,005
2,922
Total operating expenses
133,328
6,835
Loss from operations
(132,299
)
(4,471
)
Interest income
2,807
201
Net loss
$
(129,492
)
$
(4,270
)
Net loss per share, basic and diluted
$
(2.51
)
$
(0.35
)
Weighted-average shares outstanding, basic and diluted
51,544,383
12,182,478
Comprehensive loss
Net loss
$
(129,492
)
$
(4,270
)
Unrealized loss on marketable securities
(18
)
—
Comprehensive loss
$
(129,510
)
$
(4,270
)
(1) Amounts include non-cash, stock-based compensation expense as follows (in thousands):
Three Months Ended March 31,
2024
2023
Research and development
$
383
$
430
General and administrative
1,776
794
Total share-based compensation expense
$
2,159
$
1,224