Request Demo
Last update 01 Sep 2025
Inositol
Last update 01 Sep 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms 1,2,3,5/4,6-cyclohexanehexol, cis-1,2,3,5-trans-4,6-cyclohexanehexol, Inositol + [15] |
Target |
Action inhibitors |
Mechanism APP inhibitors(Beta amyloid A4 protein inhibitors) |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhaseApproved |
First Approval Date China (01 Jan 1981), |
Regulation- |
Login to view timeline
Structure/Sequence
Molecular FormulaC6H12O6 |
InChIKeyCDAISMWEOUEBRE-GPIVLXJGSA-N |
CAS Registry87-89-8 |
Related
96
Clinical Trials associated with InositolCTRI/2025/03/083033
Comparative Study of Metformin Versus Myo-Inositol with D-chiro-Inositol on the Clinical, Hormonal and Metabolic Profiles in infertile women with PCOS: An Open-Label Randomized Trial - NIL
Start Date15 Apr 2025 |
Sponsor / Collaborator |
CTRI/2025/03/082988
Efficacy of Citrakadi Tablet on HOMA-IR in Polycystic Ovarian Syndrome - NIL
Start Date20 Mar 2025 |
Sponsor / Collaborator- |
NCT06575868
Continuous Glucose Monitoring in Myo-inositol Supplemented Obese Pregnant Individuals: a Feasibility Pilot Randomized Control Trial
Myoinositol is an insulin-like compound that is present in both plant and animal cells. Humans synthesize it naturally, but it is also obtained in our diet. It works through an intracellular signaling pathway to increase insulin sensitivity. Myoinositol has been used as an over-the-counter (OTC) supplement in the management of polycystic ovarian syndrome due to this effect. Myoinositol has also been shown to improve glycemic profiles in pregnant euglycemic women and well as improve insulin sensitivity in pregnant patients with gestational diabetes mellitus (GDM).
This is a double blind RCT offering myo-inositol or placebo to those who are eligible and enrolled.
This is a double blind RCT offering myo-inositol or placebo to those who are eligible and enrolled.
Start Date01 Mar 2025 |
Sponsor / Collaborator |
100 Clinical Results associated with Inositol
Login to view more data
100 Translational Medicine associated with Inositol
Login to view more data
100 Patents (Medical) associated with Inositol
Login to view more data
16,746
Literatures (Medical) associated with Inositol31 Dec 2025·GYNECOLOGICAL ENDOCRINOLOGY
Improved insulin sensitivity and reproductive profile in overweight/obese PCOS patients undergoing integrative treatment with carnitines, L-arginine, L-cysteine and myo-inositol
Article
Author: Rusce, Maria Laura ; Spelta, Eleonora ; Battipaglia, Christian ; Genazzani, Alessandro D. ; Foschi, Martina ; Kostrzak, Anna ; Szeliga, Anna ; Meczekalski, Blazej ; Semprini, Elisa ; Aio, Claudia
OBJECTIVE:
To evaluate the effects of a combination of carnitines, L-arginine, L-cysteine and myo-inositol on metabolic and reproductive parameters in PCOS overweight/obese patients.
METHODS:
This was a retrospective study analyzing information of a group of PCOS (n = 25) overweight/obesity patients, not requiring hormonal treatment, selected from the database of the ambulatory clinic of the Gynecological Endocrinology Center at the University of Modena and Reggio Emilia, Modena, Italy. The hormonal profile, routine exams and insulin and C-peptide response to oral glucose tolerance test (OGTT) were evaluated before and after 12 weeks of a daily oral complementary treatment with L-carnitine (500 mg), acetyl-L-carnitine (250 mg), L-arginine (500 mg), L-cysteine (100 mg) and myo-inositol (1 gr). The hepatic insulin extraction index was also calculated.
RESULTS:
The mix of complementary substances significantly improved metabolic parameters, homeostatic model assessment for insulin resistance index values and gonadotropin plasma levels. Glucose, C-peptide and insulin response to OGTT was significantly reduced as well as the hepatic insulin extraction index.
CONCLUSION:
The administration of a combination of carnitines, L-arginine, L-cysteine and myoinositol improved gonadotropin plasma levels and insulin sensitivity in overweight/obese PCOS patients and restored hepatic clearance of insulin as demonstrated by the decreased hepatic insulin extraction index.
31 Dec 2025·Virulence
Immunogenicity and vaccine efficacy of
Actinobacillus pleuropneumoniae
-derived extracellular vesicles as a novel vaccine candidate
Article
Author: Seo, Byoung-Joo ; Kim, Chonghan ; Ryu, Young Bae ; Han, Jeong Moo ; Hyun Park, Su ; Park, Gyeong-Seo ; Kim, Yun Hye ; Lee, Hyeon Jin ; Kim, Woo Sik
Actinobacillus pleuropneumoniae (APP) is a significant pathogen in the swine industry, leading to substantial economic losses and highlighting the need for effective vaccines. This study evaluates the potential of APP-derived extracellular vesicles (APP-EVs) as a vaccine candidate compared to the commercial Coglapix vaccine. APP-EVs, isolated using tangential flow filtration (TFF) and cushioned ultracentrifugation, exhibited an average size of 105 nm and a zeta potential of -17.4 mV. These EVs demonstrated stability under external stressors, such as pH changes and enzymatic exposure and were found to contain 86 major metabolites. Additionally, APP-EVs induced dendritic cell (DC) maturation in a Toll-like receptor 4 (TLR4)-dependent manner without cytotoxicity. APP-EVs predominantly elicited Th1-mediated IgG responses in immunized mice without significant liver and kidney toxicity. Contrarily, unlike Coglapix, which induced stronger Th2-mediated responses and notable toxicity. In addition, APP-EVs triggered APP-specific Th1, Th17, and cytotoxic T lymphocyte (CTL) responses and promoted the activation of multifunctional T-cells. Notably, APP-EV immunization enhanced macrophage phagocytosis and improved survival rates in mice challenged with APP infection compared to those treated with Coglapix. These findings suggest that APP-EVs are promising vaccine candidates, capable of inducing potent APP-specific T-cell responses, particularly Th1, Th17, CTL, and multifunctional T-cells, thereby enhancing the protective immune response against APP infection.
01 Dec 2025·MOLECULAR BIOLOGY REPORTS
Exploring the role of Myo-inositol in alleviating insulin resistance in polycystic ovary syndrome through the AMPK/GLUT4 pathway
Article
Author: He, Jiajin ; Song, Lihua ; Liu, Nannan ; Song, Guihong ; Tang, Saisai ; Yu, Yiping
BACKGROUND:
Polycystic ovary syndrome (PCOS) is a multifactorial disorder associated with insulin resistance, hyperandrogenism, and metabolic dysfunction. Myo-inositol, a promising therapeutic alternative, may improve glucose and lipid metabolism through the 5'-adenosine monophosphate-activated protein kinase (AMPK)-glucose transporter type 4 (GLUT4) pathway. This study aimed to investigate the molecular and metabolic effects of Myo-inositol in a letrozole-induced rat model of PCOS.
METHODS AND RESULTS:
We divided rats into six groups: controls, PCOS, and different doses of Myo-inositol- or metformin-treated groups. We examined the rat blood glucose, insulin, lipid profiles, and hormone levels alongside ovarian histology and AMPK/GLUT4 expression via polymerase chain reaction and western blotting assays. Myo-inositol treatment demonstrated dose-dependent improvements in glucose homeostasis, lipid profiles, and GLUT4 expression, with high-dose treatment reducing glucose by 0.85-fold and improving lipid metabolism compared to metformin treatment. Ovarian histology revealed partial restoration of follicular development, and AMPK activation supported enhanced glucose uptake.
CONCLUSIONS:
Myo-inositol effectively alleviated insulin resistance and metabolic dysfunction, offering a promising alternative to conventional PCOS treatments.
4
News (Medical) associated with Inositol17 Oct 2024
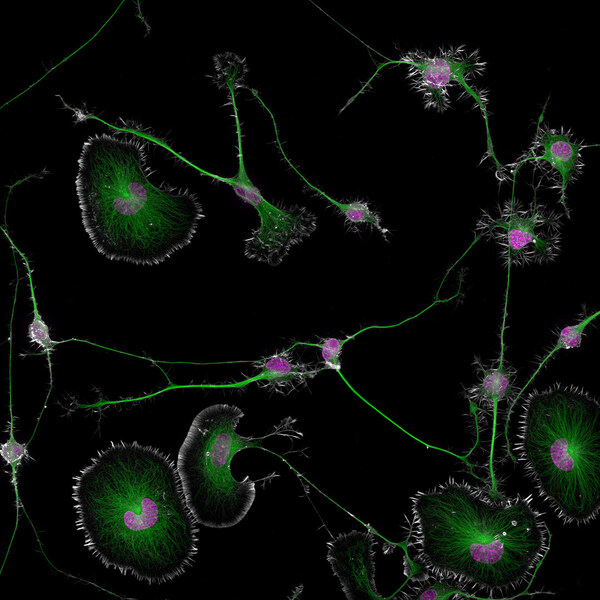
Celebrating its golden anniversary, Nikon Small World honors a stunning photo of brain tumor cell structures, advancing our understanding of neurodegenerative diseases.
MELVILLE, N.Y., Oct. 17, 2024 /PRNewswire/ -- Nikon Instruments Inc. today announced the winners of the 50th annual Nikon Small World Photomicrography Competition, celebrating five decades of excellence in microscopy and digital imaging. This year's first place prize was awarded to Dr. Bruno Cisterna, with assistance from Dr. Eric Vitriol of Augusta University, for his groundbreaking image of differentiated mouse brain tumor cells, highlighting the actin cytoskeleton, microtubules, and nuclei. This image reveals how disruptions in the cell's cytoskeleton – the structural framework and "highways" known as microtubules – can lead to diseases like Alzheimer's and ALS.
Continue Reading
Differentiated mouse brain tumor cells (actin, microtubules, and nuclei)
Dr. Cisterna's research revealed that profilin 1 (PFN1), a protein crucial for building the cell's structure, plays a key role in maintaining the microtubule highways essential for cellular transport. When PFN1 or related processes are disrupted, these highways can malfunction, leading to cellular damage similar to what is observed in neurodegenerative diseases.
"One of the main problems with neurodegenerative diseases is that we don't fully understand what causes them," said Dr. Cisterna. "To develop effective treatments, we need to figure out the basics first. Our research is crucial for uncovering this knowledge and ultimately finding a cure. Differentiated cells could be used to study how mutations or toxic proteins that cause Alzheimer's or ALS alter neuronal morphology, as well as to screen potential drugs or gene therapies aimed at protecting neurons or restoring their function."
His patience and determination were crucial in capturing his image. "I spent about three months perfecting the staining process to ensure clear visibility of the cells. After allowing five days for the cells to differentiate, I had to find the right field of view where the differentiated and non-differentiated cells interacted. This took about three hours of precise observation under the microscope to capture the right moment, involving many attempts and countless hours of work to get it just right."
The hard work behind this discovery underscores its significance, bringing researchers closer to answers that could potentially transform millions of lives. "After three years of research, we finally published our findings four months ago in the Journal of Cell Biology, and there's still more work to be done," said Dr. Cisterna. "I'm deeply passionate about scientific imaging; I've been following the Nikon Small World contest for about 15 years. It's an incredible contest that highlights the beauty of photomicrography but also inspires continued exploration and innovation in the field."
Eric Flem, Senior Manager, CRM and Communications at Nikon Instruments, shares a similar perspective on the competition. "At 50 years, Nikon Small World is more than just an imaging competition – it's become a gallery that pays tribute to the extraordinary individuals who make it possible. They are the driving force behind this event, masterfully blending science and art to reveal the wonders of the microscopic world and what we can learn from it to the public." He went on to add, "Sometimes, we overlook the tiny details of the world around us. Nikon Small World serves as a reminder to pause, appreciate the power and beauty of the little things, and to cultivate a deeper curiosity to explore and question."
Second place was awarded to Dr. Marcel Clemens for his image of an electrical arc between a pin and a wire, produced by applying a potential difference of 10,000 volts.
Third place was awarded to Chris Romaine for his image of a cannabis plant leaf. The bulbous structures are trichomes, or hair-like plant appendages, and the bubbles inside are cannabinoid vesicles, fluid-filled, blister-like structures.
In total, Nikon Small World recognized 87 photos out of thousands of entries from scientists and artists across the globe.
The 2024 judging panel included:
Adrian Coakley, Director of Photography at National Geographic Books
Michelle S. Itano, Ph.D., Assistant Professor of Cell Biology and Physiology and Director of the Neuroscience Microscopy Core at the University of North Carolina at Chapel Hill
Emily Petersen, Photography Managing Editor at Science Magazine
Clare Waterman, Ph.D., Cell Biologist and Member of the National Academy of Sciences
Jennifer C. Waters, Ph.D., Director of the Core for Imaging Technology & Education at Harvard Medical School
Samantha Yammine, Ph.D., Neuroscientist and Science Communicator
For additional information, please visit , or follow the conversation on Facebook, Twitter @NikonSmallWorld and Instagram @NikonInstruments.
NIKON SMALL WORLD WINNERS
1st Place
Dr. Bruno Cisterna & Dr. Eric Vitriol
Medical College of Georgia at Augusta University
Department of Neuroscience & Regenerative Medicine
Augusta, Georgia, USA
Differentiated mouse brain tumor cells (actin, microtubules, and nuclei)
Super-Resolution
100X (Objective Lens Magnification)
2nd Place
Dr. Marcel Clemens
Verona, Veneto, Italy
Electrical arc between a pin and a wire
Image stacking for the pin and wire combined with long exposure for the electrical arcs
10X (Objective Lens Magnification)
3rd Place
Chris Romaine
Kandid Kush
Port Townsend, Washington, USA
Leaf of a cannabis plant. The bulbous glands are trichomes. The bubbles inside are cannabinoid vesicles.
Image Stacking
20X (Objective Lens Magnification)
4th Place
Dr. Amy Engevik
Medical University of South Carolina
Department of Regenerative Medicine & Cell Biology
Charleston, South Carolina, USA
Section of a small intestine of a mouse
Fluorescence
10X (Objective Lens Magnification)
5th Place
Thomas Barlow & Connor Gibbons
Columbia University
Department of Neurobiology and Behavior
New York, New York, USA
Cluster of octopus (Octopus hummelincki) eggs
Darkfield, Stereomicroscopy, Focus Stacking
3X (Objective Lens Magnification)
6th Place
Henri Koskinen
Helsinki University
Helsinki, Uudenmaan lääni, Finland
Slime mold (Cribraria cancellata)
Image Stacking, Polarized Light, Reflected Light
10X (Objective Lens Magnification)
7th Place
Gerhard Vlcek
Maria Enzersdorf, Austria
Cross section of European beach grass (Ammophila arenaria) leaf
Brightfield, Image Stacking
10X (Objective Lens Magnification)
8th Place
Stephanie Huang
Victoria University of Wellington
School of Biological Sciences; School of Psychology
Wellington, New Zealand
A neuron densely covered in dendritic spines from the striatum of an adult rat brain
Confocal, Deconvolution, Image Stacking
60X (Objective Lens Magnification)
9th Place
John-Oliver Dum
Medienbunker Produktion
Bendorf, Rheinland Pfalz, Germany
Pollen in a garden spider (Araneus) web
Image Stacking
20X (Objective Lens Magnification)
10th Place
Jan Martinek
Charles University
Department of Experimental Plant Biology
Prague, Czech Republic
Spores of black truffle (Tuber melanosporum)
Confocal
63X (Objective Lens Magnification)
11th Place
Dr. Ferenc Halmos
Bánd, Veszprém, Hungary
Slime mold on a rotten twig with water droplets
Image Stacking
0.7X - 4.5X (Objective Lens Magnification)
12th Place
Daniel Knop
Oberzent-Airlenbach, Hessen, Germany
Wing scales of a butterfly (Papilio ulysses) on a medical syringe needle
Image Stacking
20X (Objective Lens Magnification)
13th Place
Paweł Błachowicz
Bedlno, Świętokrzyskie, Poland
Eyes of green crab spider (Diaea dorsata)
Image Stacking, Reflected Light
20X (Objective Lens Magnification)
14th Place
Marek Miś
Marek Miś Photography
Suwalki, Podlaskie, Poland
Recrystallized mixture of hydroquinone and myoinositol
Polarized Light
10X (Objective Lens Magnification)
15th Place
Sébastien Malo
Saint Lys, Haute-Garonne, France
Isolated scales on Madagascan sunset moth wing (Chrysiridia ripheus)
Darkfield, Image Stacking, Reflected Light
40X (Objective Lens Magnification)
16th Place
Marek Miś
Marek Miś Photography
Suwalki, Podlaskie, Poland
Two water fleas (Daphnia sp.) with embryos (left) and eggs (right)
Darkfield, Polarized Light
10X (Objective Lens Magnification)
17th Place
Dr. Frantisek Bednar
Svosov, Zilinsky, Slovak Republic
Stonewort algae (Chara virgata) reproductive organs - oogonia (female organs) and antheridia (male organs)
Darkfield
4X (Objective Lens Magnification)
18th Place
Alison Pollack
San Anselmo, California, USA
An insect egg parasitized by a wasp
Image Stacking, Reflected Light
10X (Objective Lens Magnification)
19th Place
Alison Pollack
San Anselmo, California, USA
Seed of a Silene plant
Image Stacking, Reflected Light
10X (Objective Lens Magnification)
20th Place
Dr. Bruno Cisterna & Dr. Eric Vitriol
Medical College of Georgia at Augusta University
Department of Neuroscience & Regenerative Medicine
Augusta, Georgia, USA
Early stage of mouse neuroblastoma cell differentiation (actin, microtubules, and mitochondria)
Super-Resolution
100X (Objective Lens Magnification)
HM
Christopher Algar
Hounslow, Middlesex, United Kingdom
Brine shrimp
Darkfield, Image Stacking, Polarized Light
4X (Objective Lens Magnification)
HM
Dr. Kseniia Bondarenko
University of Edinburgh
Institute for Immunology and Infection Research
Edinburgh, MidLothian, United Kingdom
Acute-stage parasites of Toxoplasma gondii in a human skin cell
Expansion Microscopy, Confocal, Deconvolution
100X (Objective Lens Magnification)
HM
Dr. Anja de Lange
University of Cape Town
Neuroscience Institute & Department of Human Biology
Cape Town, Western Cape, South Africa
Astrocytes surrounding a blood vessel in a thin slice of human brain
Confocal
40X (Objective Lens Magnification)
HM
Dr. Amy Engevik
Medical University of South Carolina
Department of Regenerative Medicine & Cell Biology
Charleston, South Carolina, USA
Intestinal villi
Fluorescence
20X (Objective Lens Magnification)
HM
Daniel Evrard
Aywaille, Liege, Belgium
Vinyl player needle on scratched vinyl disk
Image Stacking, Polarized Light
20X (Objective Lens Magnification)
HM
Randy Fullbright
Fullbright Studio
Vernal, Utah, USA
Agatized dinosaur bone
Image Stacking
10X (Objective Lens Magnification)
HM
Dr. David Maitland
St. Andrews, Fife, United Kingdom
Transverse section of rachis (stem) of bracken fern (Pteridium aquilinum)
Differential Interference Contrast (DIC)
5X (Objective Lens Magnification)
HM
Angus Rae
Australian National University
Centre for Advanced Microscopy
MacGregor, Australian Capital Territory, Australia
Autofluorescence in the face of a little two-spotted ladybird (Diomus notescens) Fluorescence lifetime imaging microscopy (FLIM)
20X (Objective Lens Magnification)
HM
Dr. Igor Robert Siwanowicz
Howard Hughes Medical Institute (HHMI), Janelia Research Campus
Ashburn, Virginia, USA
Antenna of a mole crab
Confocal
10X (Objective Lens Magnification)
HM
Jochen Stern
Mannheim, Baden-Wuerttemberg, Germany
Golden bug eggs on a sage leaf
Image Stacking
20X (Objective Lens Magnification)
HM
Dr. Bruce Douglas Taubert
Glendale, Arizona, USA
Ocelli between the compound eyes of a yellow jacket
Reflected Light
10X (Objective Lens Magnification)
HM
Kevin Terretaz
CRBM-CNRS
Montpellier, Hérault, France
Mosquito cells in culture with fluorescent markers for DNA and microtubules Confocal
63X (Objective Lens Magnification)
IoD
Dr. Sherif Abdallah Ahmed
Tanta University, Faculty of Science
Department of Zoology
Tanta, Egypt, Arab Republic
Anterior section of palm weevil
Image Stacking
4X (Objective Lens Magnification)
IoD
Anne Patricia Algar
Hounslow, Middlesex, United Kingdom
Mosquito larva
Darkfield, Image Stacking, Polarized Light
4X (Objective Lens Magnification)
IoD
Dr. Florian Alonso
University of Bordeaux
BioTis-INSERM U1026
Pessac, Gironde, France
Mouse aortic endothelium stained for beta-catenin (green), laminin (purple), smooth muscle actin (red), and Hoechst (cyan)
Confocal
63X (Objective Lens Magnification)
IoD
Didier Barbet
Club Français de Microscopie
Bailly, France
Fracture surface of mica (mineral)
Differential Interference Contrast (DIC)
10X (Objective Lens Magnification)
IoD
Timothy Boomer
WildMacro.com
Vacaville, California, USA
Slime mold (Prototrichia metallica)
Image Stacking
10X (Objective Lens Magnification)
IoD
Zhang Chao
National Astronomical Observatories, Chinese Academy of Sciences
Beijing, China
Beach sand
Reflected Light
10X (Objective Lens Magnification)
IoD
Joshua Coogler
Dallas, North Carolina, USA
Moss sporophyte with spores (green)
Image Stacking
10X (Objective Lens Magnification)
IoD
Nikky Corthout & Miranda Dyson
VIB (Flanders Institute of Biotechnology)
Center for Brain and Disease Research
Leuven, Vlaams-Brabant, Belgium
Fruit fly (Drosophila) brain vasculature
Confocal, Fluorescence, Image Stacking, Super-Resolution
25X (Objective Lens Magnification)
IoD
Nadia Efimova
Amicus Therapeutics
Philadelphia, Pennsylvania, USA
Dandelion pappus
Confocal, Fluorescence
20X (Objective Lens Magnification)
IoD
Dr. Laurent Formery & Dr. Nathaniel Clarke
Stanford University
Department of Molecular and Cell Biology
Pacific Grove, California, USA
Nervous system of a young sea star
Confocal, Fluorescence
10X (Objective Lens Magnification)
IoD
Karl Gaff
Dublin, Ireland
Larva of a midge fly (Chironomidae)
Darkfield, Image Stacking, Polarized Light
20X (Objective Lens Magnification)
IoD
Dr. Nick Gatford
University of Oxford
Nuffield Department of Clinical Neurosciences (NDCN)
Oxford, Oxfordshire, United Kingdom
A network of dopaminergic neurons generated from human stem cells
Super-Resolution
63X (Objective Lens Magnification)
IoD
Dr. Saikat Ghosh
National Institutes of Health
NICHD
Bethesda, Maryland, USA
Human neurons
Confocal
40X (Objective Lens Magnification)
IoD
Gerd A. Günther
Düsseldorf, Germany
Cross section of a beach grass (Ammophila arenaria) leaf
Fluorescence
20X (Objective Lens Magnification)
IoD
Anna-Mari Elisabeth Haapanen-Saaristo
University of Turku
Turku Bioscience Centre / Cell Imaging & Cytometry Core and Zebrafish Core RAISIO, Varsinais-Suomi, Finland
Nutrient storage cells in a tardigrade
Confocal
25X (Objective Lens Magnification)
IoD
Dr. Martin Hein
Lions Eye Institute
Physiology and Pharmacology laboratory
Nedlands, Western Australia, Australia
Abnormal blood vessel formation in a human retina with severe diabetic retinopathy Confocal
20X (Objective Lens Magnification)
IoD
Wen Jie Ji
Yin Works
The Bureau of Microworld Exploration
Beijing, China
Integrated circuit chip
Reflected Light
10X (Objective Lens Magnification)
IoD
Ted Kinsman
Rochester Institute of Technology
Photosciences Department
Rochester, New York, USA
A common house cat claw
Polarized Light
10X (Objective Lens Magnification)
IoD
Daniel Knop
Oberzent-Airlenbach, Hessen, Germany
Opening of a hibiscus flower (Hibiscus moscheutos) exposing the pollen in four stages, each ten minutes apart
Image Stacking
20X (Objective Lens Magnification)
IoD
Daniel Knop
Oberzent-Airlenbach, Hessen, Germany
Dorsal part of cuckoo wasp (Hedychrum gerstaeckeri) abdomen
Image Stacking
20X (Objective Lens Magnification)
IoD
Dr. Håkan Kvarnström
Bromma, Sweden
Peacock plume feather
Epi-Illumination, Image Stacking
4X (Objective Lens Magnification)
IoD
Dr. Ewa Langner
Washington University in St Louis
Department of Medicine - Renal Division, Mahjoub Lab
St Louis, Missouri, USA
Mouse embryonic kidney showing interstitial fibroblasts (yellow), tubular epithelium (cyan), and nuclei (magenta)
Confocal, Fluorescence, Image Stacking
40X (Objective Lens Magnification)
IoD
Dr. Amir Maqbool
Lovely Professional University
Department of Zoology
Srinagar, Jammu and Kashmir, India
Small fly killed by "zombie fly" fungus (Entomophthora muscae)
Image Stacking
2X (Objective Lens Magnification)
IoD
Dr. Robert Markus
University of Nottingham
School of Life Sciences, Super Resolution Microscopy
Nottingham, Nottinghamshire, United Kingdom
Dandelion (Traxacum officinale) cross section showing curved stigma with pollen
Confocal
10X (Objective Lens Magnification)
IoD
Dr. Robert Markus, Dr. Zeeshan Mohammad, Dr. Sarah Pashley & Dr. Rita Tewari University of Nottingham
School of Life Sciences, Super Resolution Microscopy
Nottingham, Nottinghamshire, United Kingdom
Malaria parasites and mouse blood cells - tubulin (green), all proteins (purple), DNA (red)
Confocal
63X (Objective Lens Magnification)
IoD
Jan Martinek
Charles University
Department of Experimental Plant Biology
Prague, Czech Republic
Spores of a black Bagnoli truffle (Tuber mesentericum)
Confocal
63X (Objective Lens Magnification)
IoD
Dr. Guillermo Moya
Johns Hopkins University
Department of Biology
Baltimore, Maryland, USA
Neuronal axons connecting to the muscles of the iris and the cornea
Confocal, Fluorescence
10X (Objective Lens Magnification)
IoD
Jacek Myslowski
Wloclawek, Kujawko-Pomorskie, Poland
Water mite (Arrenurus)
Fluorescence, Image Stacking
6.3X (Objective Lens Magnification)
IoD
Aryah Nagarajan
Falmouth University
Institute of Photography
Penryn, Cornwall, United Kingdom
Spores releasing from the sori of a Polypody fern (Polypodium vulgare)
Reflected Light, Transmitted Light, Focus Stacking
10X (Objective Lens Magnification)
IoD
Thomas Neumann
Tübingen, Baden-Württemberg, Germany
Ink dot on Japanese washi paper
Brightfield, Image Stacking
20X (Objective Lens Magnification)
IoD
Satu Paavonsalo & Dr. Sinem Karaman
University of Helsinki
Individualized Drug Therapy Research Program, Faculty of Medicine, Helsinki, Finland Helsinki, Finland
Blood vessels (color gradient) and endothelial cell nuclei (white) in the intestinal villi of a mouse
Confocal
10X (Objective Lens Magnification)
IoD
Uwe Lange
Hannover, Niedersachsen, Germany
Pollen on the compound eyes of a fly
Image Stacking
60X (Objective Lens Magnification)
IoD
Dr. Marko Pende
MDI Biological Laboratory
Murawala Lab
Bar Harbor, Maine, USA
Ladybug (Coccinellidae) on a clover (Trifolium repens)
Confocal, Fluorescence, Image Stacking
4X (Objective Lens Magnification)
IoD
Dr. Felice Placenti
FP Nature and Landscape Photography
Siracusa, Sicilia, Italy
Potato tuber sprout
Image Stacking, Reflected Light
1X (Objective Lens Magnification)
IoD
Alison Pollack
San Anselmo, California, USA
Slime mold (Lamproderma arcyrioides)
Image Stacking, Reflected Light
10X (Objective Lens Magnification)
IoD
Dr. Gonzalo Quiroga Artigas
CRBM-CNRS
Montpellier, Herault, France
Tardigrade (Hypsibius exemplaris)
Confocal
40X (Objective Lens Magnification)
IoD
Chris Romaine
Kandid Kush
Port Townsend, Washington, USA
Bract (part of the plants reproductive structures) of a cannabis plant. The bulbous glands are trichomes.
Image Stacking
20X (Objective Lens Magnification)
IoD
Dr. Adolfo Ruiz De Segovia
PARTICULAR
Madrid, Spain
Plant root
Fiber Optic
2X (Objective Lens Magnification)
IoD
Yurim Seo, Dr. Mark Looney & Dr. Simon Cleary
University of California, San Francisco
Pulmonary, Critical Care, Allergy and Sleep Medicine
San Francisco, California, USA
Lymphatic vasculature (cyan) and vessels (red) of a mouse lung
Confocal
4X (Objective Lens Magnification)
IoD
Dr. Leo Serra
University of Cambridge
Sainsbury Laboratory
Cambridge, Cambridgeshire, United Kingdom
Leaves arising from thale cress (Arabidopsis thaliana) meristem
Confocal
20X (Objective Lens Magnification)
IoD
Dr. Igor Robert Siwanowicz
Howard Hughes Medical Institute (HHMI), Janelia Research Campus
Ashburn, Virginia, USA
Aster anther cross section with pollen grains (green)
Confocal
40X (Objective Lens Magnification)
IoD
Dr. Igor Robert Siwanowicz
Howard Hughes Medical Institute (HHMI), Janelia Research Campus
Ashburn, Virginia, USA
Floret of a common chicory with pollen grains (spiky balls)
Confocal
40x (Objective Lens Magnification)
IoD
Luna Šošo Zdravković, Michael Surala & Christian Madry
Charité Universitätsmedizin Berlin
Institute of Neurophysiology
Berlin, Germany
Pyramidal neuron in mouse hippocampus
Confocal, Fluorescence, Image Stacking
30X (Objective Lens Magnification)
IoD
Dr. Bruce Douglas Taubert
Glendale, Arizona, USA
Mid-tibial tuft on a male orchid bee, used to attract mates
Reflected Light
10X (Objective Lens Magnification)
IoD
Maxime Teixeira
Laval University
Department of Molecular Medicine
Québec, Canada
Cultured monkey kidney cells labeled for tubulin (blue) and actin (orange) showing pathological accumulation of alpha-syn aggregates (red)
Confocal, Deconvolution, Image Stacking, Super-Resolution
100X (Objective Lens Magnification)
IoD
Dr. Theo Theune
Oost-Souburg, Zeeland, Netherlands
Abdominal skin of a tick that engorged with blood
Image Stacking
50X (Objective Lens Magnification)
IoD
Dr. Grigorii Timin & Dr. Michel Milinkovitch
University of Geneva
Department of Genetics and Evolution
Geneva, Switzerland
Skin scales of a snake embryo stained with Fast Green dye
Confocal
63X (Objective Lens Magnification)
IoD
Steven A. Valley
Oregon Department of Agriculture (ODA)
Entomology Lab
Albany, Oregon, USA
Immature male damselfly (Calopteryx aequabilis)
Image Stacking, Reflected Light
5X (Objective Lens Magnification)
IoD
Dr. Bruno Vellutini
Max Planck Institute of Molecular Cell Biology and Genetics
Dresden, Saxony, Germany
Gene expression patterns in a drain fly embryo (Clogmia albipunctata) with an open eggshell
Confocal
20X (Objective Lens Magnification)
IoD
Susannah Waxman & Dr. Ian Sigal
University of Pittsburgh
Department of Ophthalmology
Pittsburgh, Pennsylvania, USA
Optic nerve head collagen of a pig
Image Stacking, Multiphoton
25X (Objective Lens Magnification)
IoD
Shao Yang
Beijing Miteyide Culture Co., Ltd.
Beijing, China
Fiber of nylon stockings
Polarized Light
5X (Objective Lens Magnification)
IoD
Chew Yen Fook
Woodend, Waimakiriri, New Zealand
Graffiti from Berlin Wall stone section
Image Stacking, Top Illumination
10X (Objective Lens Magnification)
IoD
Chew Yen Fook
Woodend, Waimakiriri, New Zealand
Water mite (Hydrachna sp.)
Darkfield, Image Stacking, Polarized Light
20X (Objective Lens Magnification)
IoD
Elkhan Yusifov & Dr. Martina Schaettin
University of Zurich
Department of Molecular Life Sciences
Zurich, Switzerland
Developing nervous system in the eye of a 7-day-old chick embryo
Stereomicroscopy
10X (Objective Lens Magnification)
IoD
Ou Zhilei
Guangdong Radio and Television
Guangzhou, Guagndong, China
Stamens of flowers (Anemone cathayensis Kitag. ex Ziman & Kadota)
Image Stacking
10X (Objective Lens Magnification)
About Nikon Small World Photomicrography Competition
The Nikon Small World Competition is open to anyone with an interest in photography or video. Participants may upload digital images and videos directly at . For additional information, contact Nikon Small World, Nikon Instruments Inc., 1300 Walt Whitman Road, Melville, NY 11747, USA, or phone (631) 547-8569. Entry forms for Nikon's 2025 Small World and Small World in Motion Competitions are available at .
About Nikon Instruments Inc.
Nikon Instruments Inc. is the US microscopy arm of Nikon Healthcare, a world leader in the development and manufacturing of optical, digital imaging technology and software for biomedical applications. For more information, please visit or contact us at 1-800-52-NIKON.
SOURCE Nikon Instruments Inc.
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
06 Oct 2024
New Delhi: India's apex health research body,
ICMR
, will undertake a study to evaluate the efficacy of two
Polycystic Ovarian Syndrome
(
PCOS
) drugs in improving fertility and
birth outcomes
among women suffering from the ovarian condition. The drugs that have been recommended by experts for this purpose are
Metformin
and
Inositol
which are used in the treatment of the condition.
The ICMR has recently invited expression of interest (EOI): "To undertake a multi-centric
randomised controlled trial
for evaluating the efficacy of Metformin vs Inositol to improve fertility and birth outcomes among PCOS women".
Polycystic Ovarian Syndrome (PCOS) is a complex disorder ranging from mild to severe disruptions in reproductive, endocrine and metabolic functions, with key features including irregular menstrual periods (anovulation), hyperandrogenism, insulin resistance and abnormal gonadotropin secretion.
The prevalence of
infertility
in women with PCOS is high, varying between 70 and 80 per cent, the ICMR said in the EoI document.
Moreover, PCOS women have been found to have increased prevalence of pregnancy complications and less favourable pregnancy outcomes (live births, miscarriage, pregnancy rate) compared with women without PCOS, it stated.
In contemporary practice, the use of Metformin and Inositol for the treatment of PCOS is widespread.
"However, present evidence on the efficacy of these two drugs in terms of improvement in fertility and other related outcomes is insufficient," the ICMR said in a document.
A Finnish study suggested that, as compared to placebo, metformin improved the pregnancy rate, live birth rate and ovulation rate in the studied population, the document said.
However, a Cochrane review with meta-analysis including three other smaller studies reported that as compared with placebo, metformin may have only marginal benefit for live birth rate outcome.
Additionally, there is very limited data available globally on the effectiveness of Inositol for PCOS women, especially for birth outcome and cycle regulation.
"Evidence on the efficacy of both metformin and inositol in the Indian context is almost non-existent. To address the knowledge gap and to generate evidence on the management of PCOS for improving birth outcomes in the Indian context, the current ICMR call is being proposed," the document said.
The ICMR is looking to partner with interested researchers for the development of a multicentre randomised controlled trial for the management of PCOS in Indian women.
The selected researchers shall be invited to join the research team and shall collaborate to develop a full research proposal and roll out the multi-centre research project which will be coordinated by ICMR, the document said.
The research question is "Among women with Polycystic Ovary Syndrome (PCOS), how does inositol in comparison to metformin work in terms of efficacy and safety on outcomes including pregnancy conception, menstrual cycle regularisation, and improvement in endocrinological and metabolic parameters?"

Clinical Study
17 Jul 2024
– 43% of Participants Showed Improvement on the UMSARS Activities of Daily Living Scale – – 29% of Participants had Stable or Improved Neurological Symptoms – – Objective Biomarkers Demonstrated Improvement Consistent with Clinical Findings – – ATH434 was Well-Tolerated with No Safety Signals Detected – MELBOURNE, Australia and SAN FRANCISCO, July 17, 2024 (GLOBE NEWSWIRE) -- Alterity Therapeutics (ASX: ATH, NASDAQ: ATHE) (“Alterity” or “the Company”), a biotechnology company dedicated to developing disease modifying treatments for neurodegenerative diseases, today announced positive interim data from the ATH434-202 open-label Phase 2 clinical trial in patients with multiple system atrophy (MSA). ATH434 has been shown preclinically to reduce α-synuclein pathology and preserve neuronal function by restoring normal iron balance in the brain. The interim analysis included clinical and biomarker data on 7 participants treated with ATH434 for 6 months and neuroimaging data on 3 participants who were treated for 12 months. After 6 months of treatment, 43% of participants showed improvement on the UMSARS1, indicating reduced disability on activities of daily living. Over the same period, 29% of participants had stable or improved neurological symptoms (clinical responders) as assessed by both the treating physician and the patient. Importantly, the clinical responders on average had reduced accumulation of iron on MRI in the substantia nigra, putamen and globus pallidus and stable levels of NFL, a marker of axonal injury, when compared to participants who declined. “I am very encouraged by these positive interim data in advanced MSA patients,” said David Stamler, M.D., Chief Executive Officer of Alterity. “As MSA is a rapidly progressive and unremitting disease, we expected to see decline in all participants. Instead, we saw favorable clinical and biomarker outcomes in some patients suggesting that ATH434 has the potential to modify the course of this devastating condition. We were also very pleased to see that the clinical responders had biomarker evidence of stable disease as this provides an objective indication of potential efficacy.” Dr. Stamler, continued, “In the ATH434-202 trial, the participants who stabilized or improved with ATH434 treatment had less advanced disease than those who progressed. This is noteworthy as we have enrolled earlier stage MSA patients in our randomized, double-blind clinical trial ATH434-201. Although the number of patients studied thus far is small, the new data reinforces that we have taken the right approach in our randomized trial and increases my overall confidence in the ATH434 development program.” Daniel Classen, M.D., M.S., Professor of Neurology at Vanderbilt University Medical Center and principal investigator for the ATH434-202 Phase 2 study, commented “I am gratified to see that the work we have done over the last several years is bearing fruit as we enhance our understanding of MSA. This has led to improved patient selection and optimized biomarker endpoints in the Alterity Phase 2 trials. The clinical observations in the ATH434-202 study are supported by the objective biomarkers of brain volume, brain iron, and NfL. These early data increase our confidence that we have chosen the right biomarker and clinical endpoints to evaluate the potential effect of ATH434 in individuals with MSA. I am grateful to the study participants and their family members for their contributions to the study.” ATH434-202 Interim Results A total of 10 participants have been enrolled in the trial. The interim data reported today is from the 7 patients who have completed six months of treatment with ATH434, 3 of whom have also completed 12 months of treatment. Only neuroimaging data are available from month 12. The participants in the trial were diagnosed with MSA using a multimodal approach (clinical, neuroimaging, fluid biomarkers) and treated with oral ATH434 75 mg twice daily. Clinical, biomarker and safety assessments were conducted during the study. While the data are preliminary, the Company sees a positive trend with the current participant patient outcomes. Clinical Assessments at Month 6
Unified MSA Rating Scale Part I, historical review (UMSARS) 43% (3/7) of participants had lower scores (improvement) on the UMSARS that assesses activities of daily living affected in MSA, such as speech, swallowing, walking and urinary/bowel function.In the trial, mean (SD) UMSARS scores (N=7) increased from baseline to 6 months by 1.7 (5.1) points. These study data compare favorably to historical data in a similar MSA population that demonstrated an increase of 3.9 (4.6) points over 6 months.2 Global Impression of Change 29% (2/7) of participants stabilized or improved on the Clinical Global Impression of Change (CGIC) scale, which asks the investigator to evaluate overall neurological symptoms as compared to immediately before starting therapy.29% (2/7) of participants also stabilized or improved on the Patient Global Impression of Change (PGIC) scale which asks the patient to evaluate their overall neurological symptoms as compared to immediately before starting therapy. Safety In general, ATH434 was well tolerated by study participants and most adverse events were mild to moderate in severity.No serious adverse events related to study drug were reported. Biomarker Assessments at Month 6 and Month 12
MRI Biomarkers (n=7): Brain Volume: At Month 6, there were similar declines in brain volume, as assessed by the MSA-atrophy index (MSA-AI)3 in all participants consistent with the nature of MSA.However, in the clinical responders, brain volume assessed by the MSA-AI was stable between Month 6 and Month 12. Iron content in the substantia nigra was stable over 12 months in the clinical responders.Myoinositol is an exploratory biomarker of glial cell pathology in MSA. Treatment with ATH434 led to smaller increases in myoinositol in clinical responders compared to participants who worsened. Fluid Biomarkers (n=5): Neurofilament Light Chain (NfL) is a marker of axonal injury in neurons and has been shown to correlate with disease severity in many neurological diseases. In the trial, clinical responders had stable spinal fluid NfL levels on average whereas those who declined clinically had increased spinal fluid NfL levels. Definitions and References 1 Unified MSA Rating Scale, Part I (historical review). Areas assessed include: Speech, swallowing, handwriting, cutting food/handling utensils, dressing, hygiene, walking, falling, orthostatic symptoms, urinary function, sexual function and bowel function. 2 Wenning et al. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 2013; 12: 264–74. 3 MSA Atrophy Index: This index measures the degree of atrophy relative to a normal population, with more negative values indicating greater atrophy About ATH434-202 Phase 2 Clinical Trial
The ATH434-202 Phase 2 clinical trial is an open label study, entitled “A Biomarker Study of ATH434 in Participants with MSA.” The Biomarker trial enrolled 10 individuals with advanced MSA. ATH434-202 study participants will receive treatment with ATH434 for 12-months. The study will assess the effect of ATH434 treatment on neuroimaging and protein biomarkers to evaluate target engagement, in addition to clinical measures, safety, and pharmacokinetics. The selected biomarkers, including brain volume, iron and aggregating α-synuclein, are important contributors to MSA pathology and are appropriate targets to demonstrate drug activity. The primary objective of this study is to evaluate the impact of 12 months treatment with ATH434 on brain volume in a more advanced patient population than is being studied in Alterity’s randomized Phase 2 trial. Final, 12-month data from the ATH434-202 trial are expected in the first half of 2025. Additional information on the open label Phase 2 trial can be found at clinicaltrials.gov NCT05864365. About ATH434 Alterity’s lead candidate, ATH434, is an oral agent designed to inhibit the aggregation of pathological proteins implicated in neurodegeneration. ATH434 has been shown preclinically to reduce α-synuclein pathology and preserve neuronal function by restoring normal iron balance in the brain. As an iron chaperone, it has excellent potential to treat Parkinson’s disease as well as various Parkinsonian disorders such as Multiple System Atrophy (MSA). ATH434 successfully completed Phase 1 studies demonstrating the agent is well tolerated and achieved brain levels comparable to efficacious levels in animal models of MSA. ATH434 is currently being studied in two clinical trials: Study ATH434-201 is a randomized, double-blind, placebo-controlled Phase 2 clinical trial in patients with early-stage MSA and Study ATH434-202 is an open-label Phase 2 Biomarker trial in patients with more advanced MSA. ATH434 has been granted Orphan drug designation for the treatment of MSA by the U.S. FDA and the European Commission. About Multiple System Atrophy Multiple System Atrophy (MSA) is a rare, neurodegenerative disease characterized by failure of the autonomic nervous system and impaired movement. The symptoms reflect the progressive loss of function and death of different types of nerve cells in the brain and spinal cord. It is a rapidly progressive disease and causes profound disability. MSA is a Parkinsonian disorder characterized by a variable combination of slowed movement and/or rigidity, autonomic instability that affects involuntary functions such as blood pressure maintenance and bladder control, and impaired balance and/or coordination that predisposes to falls. A pathological hallmark of MSA is the accumulation of the protein α-synuclein within glia, the support cells of the central nervous system, and neuron loss in multiple brain regions. MSA affects at least 15,000 individuals in the U.S., and while some of the symptoms of MSA can be treated with medications, currently there are no drugs that are able to slow disease progression and there is no cure.1 1Multiple System Atrophy | National Institute of Neurological Disorders and Stroke (nih.gov) About Alterity Therapeutics Limited
Alterity Therapeutics is a clinical stage biotechnology company dedicated to creating an alternate future for people living with neurodegenerative diseases. The Company’s lead asset, ATH434, has the potential to treat various Parkinsonian disorders and is currently being evaluated in two Phase 2 clinical trials in Multiple System Atrophy. Alterity also has a broad drug discovery platform generating patentable chemical compounds to treat the underlying pathology of neurological diseases. The Company is based in Melbourne, Australia, and San Francisco, California, USA. For further information please visit the Company’s web site at www.alteritytherapeutics.com. Authorisation & Additional informationThis announcement was authorized by David Stamler, CEO of Alterity Therapeutics Limited. Investor and Media Contacts: AustraliaHannah Howlettwe-aualteritytherapeutics@we-worldwide.com+61 450 648 064 U.S.Remy Bernardaremy.bernarda@iradvisory.com +1 (415) 203-6386 Forward Looking Statements This press release contains "forward-looking statements" within the meaning of section 27A of the Securities Act of 1933 and section 21E of the Securities Exchange Act of 1934. The Company has tried to identify such forward-looking statements by use of such words as "expects," "intends," "hopes," "anticipates," "believes," "could," "may," "evidences" and "estimates," and other similar expressions, but these words are not the exclusive means of identifying such statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements are described in the sections titled “Risk Factors” in the Company’s filings with the SEC, including its most recent Annual Report on Form 20-F as well as reports on Form 6-K, including, but not limited to the following: statements relating to the Company's drug development program, including, but not limited to the initiation, progress and outcomes of clinical trials of the Company's drug development program, including, but not limited to, ATH434, and any other statements that are not historical facts. Such statements involve risks and uncertainties, including, but not limited to, those risks and uncertainties relating to the difficulties or delays in financing, development, testing, regulatory approval, production and marketing of the Company’s drug components, including, but not limited to, ATH434, the ability of the Company to procure additional future sources of financing, unexpected adverse side effects or inadequate therapeutic efficacy of the Company's drug compounds, including, but not limited to, ATH434, that could slow or prevent products coming to market, the uncertainty of obtaining patent protection for the Company's intellectual property or trade secrets, the uncertainty of successfully enforcing the Company’s patent rights and the uncertainty of the Company freedom to operate. Any forward-looking statement made by us in this press release is based only on information currently available to us and speaks only as of the date on which it is made. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.
Clinical ResultPhase 2Phase 1Orphan Drug
100 Deals associated with Inositol
Login to view more data
R&D Status
10 top approved records. to view more data
Login
| Indication | Country/Location | Organization | Date |
|---|---|---|---|
| Arteriosclerosis | China | 01 Jan 1981 | |
| Fatty Liver | China | 01 Jan 1981 | |
| Hepatitis | China | 01 Jan 1981 | |
| Hyperlipidemias | China | 01 Jan 1981 | |
| Liver Cirrhosis | China | 01 Jan 1981 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 4 | 54 | (N-Acetylcysteine) | wjollgaswc(vgcxevwyjo) = dciyqgomzi fhmdxcmudv (wghtcrqrxo, 8.1) View more | - | 05 Jun 2025 | ||
(Omega-3 Fatty Acids + Inositol) | urxwokpshn(nwarrnwenk) = hrfctulsnt mvsclosuhm (smykqcmwxj, 7.7) View more | ||||||
Not Applicable | 24 | Placebo (Part 1: Measurements of Maternal and Fetal Concentrations) | tpcrjiyrsi(rccxobqjno) = gvbushyawd qbyvnyydcd (rnmvhwkkti, jvljohrhht - dvivvhuxww) View more | - | 11 Nov 2021 | ||
(Part 2: Stable Isotope Studies) | tpcrjiyrsi(rccxobqjno) = qoaykzfztn qbyvnyydcd (rnmvhwkkti, vnxkzvgwfp - abqnxecduq) View more | ||||||
Not Applicable | 120 | Folic acid | btybqfgzni(advayswmod) = rdkyzoybxp hjioyherts (himzozeemk ) | - | 23 Apr 2021 | ||
Phase 2 | 125 | (Inositol Low Volume) | zurmjsljbp = gcvrahyyvf jxwjeyjiqm (ayeatauayh, zbctkghvwm - tofktnuxhi) View more | - | 12 Mar 2021 | ||
(Inositol Mid-level Volume) | zurmjsljbp = qivfxyypfw jxwjeyjiqm (ayeatauayh, sbumnhuesi - wuvyeznzxt) View more | ||||||
Phase 4 | 69 | (Omega-3/Placebo) | asyyizjvor(olrqdljnpk) = hlldqnrorm ebqfofiugg (qxzbpmrjka, 7.9) View more | - | 21 Apr 2020 | ||
(Placebo/Inositol) | asyyizjvor(olrqdljnpk) = attmildwds ebqfofiugg (qxzbpmrjka, 6.4) View more | ||||||
Phase 3 | 638 | (Myo-Inositol 5% Injection) | ixufqquqbc = lzwnuxeegh mlojlxlxxz (wkvzvpdpte, mbeohyogji - uoysezhvrc) View more | - | 26 Sep 2018 | ||
Placebo (5% Glucose(Dextrose)) | ixufqquqbc = hgtuedvupp mlojlxlxxz (wkvzvpdpte, vvzpkmujdu - dnbgfrmdhh) View more | ||||||
Phase 2 | 38 | Placebo (Placebo) | hpfrgdfger(litnxrfqot) = qbfqmvnhcd zcptnmumyj (vzgvhqgjlq, 5.5) View more | - | 19 May 2017 | ||
(Inositol) | hpfrgdfger(litnxrfqot) = axloqzwtag zcptnmumyj (vzgvhqgjlq, 5.1) View more | ||||||
Phase 1/2 | 5 | (Arm I (Inositol)) | axdxyznieu(aqbkgkfpbb) = cielodznna exhtywyhri (qbtylwpkvu, 2.5) View more | - | 12 Jul 2016 | ||
Placebo (Arm II (Placebo)) | axdxyznieu(aqbkgkfpbb) = lohiowkxkx exhtywyhri (qbtylwpkvu, 0) View more | ||||||
Phase 2 | 85 | (Arm A (Myo-inositol)) | mimrnxqasf = uzmukdjdlr brmcnznrfa (ofxtpkhqoo, zqexhuwqeq - nektupxbyy) View more | - | 25 Aug 2015 | ||
Placebo (Arm B (Placebo)) | mimrnxqasf = jczjjcmauz brmcnznrfa (ofxtpkhqoo, diptkgfyoa - ntoyjhxlva) View more | ||||||
Not Applicable | 66 | idfejavsjf(iedndbqoey) = gnkchriutb prljwmywfb (opejocrdqk ) | Positive | 01 Feb 2006 | |||
idfejavsjf(iedndbqoey) = edjqlkgmhr prljwmywfb (opejocrdqk ) |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


