Request Demo
Last update 14 Jun 2025
MK-2060
Last update 14 Jun 2025
Overview
Basic Info
Drug Type Bispecific antibody |
Synonyms Anti-Factor XI Monoclonal Antibody, MK 2060, MK-2060 + [1] |
Target |
Action inhibitors, stimulants |
Mechanism F11 inhibitors(Coagulation factor XI inhibitors), Blood coagulation stimulants |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhasePhase 2 |
First Approval Date- |
RegulationFast Track (United States) |
Login to view timeline
Related
9
Clinical Trials associated with MK-2060NCT06582602
A Single Dose Study to Assess the Safety, Pharmacokinetics, and Pharmacodynamics of Intravenous Infusion and Intravenous Bolus Administration of MK-2060 in Healthy Participants
The goal of the study is to learn about the safety of MK-2060 and if people tolerate it when MK-2060 is given in different forms.
Start Date15 Oct 2024 |
Sponsor / Collaborator |
NCT05769595
A Single-dose Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of MK-2060 in Japanese Older Participants With End-stage Renal Disease on Dialysis.
The purpose of this study is to assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of MK-2060 after a single dose intravenous (IV) administration in Japanese older participants with end stage renal disease (ESRD) on dialysis. There is no primary hypothesis for this study.
Start Date14 Jun 2023 |
Sponsor / Collaborator |
JPRN-jRCT2031220658
A Single-Ascending Dose Clinical Study to Evaluate the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of MK-2060 in Healthy Japanese Participants
Start Date27 Mar 2023 |
Sponsor / Collaborator- |
100 Clinical Results associated with MK-2060
Login to view more data
100 Translational Medicine associated with MK-2060
Login to view more data
100 Patents (Medical) associated with MK-2060
Login to view more data
2
Literatures (Medical) associated with MK-206001 Dec 2018·BMC hematology
Rare and unusual case of anti-factor XI antibodies in patient with plasma cell leukemia
Author: Uwingabiye, Jean ; Hadef, Rachid ; El Kabbaj, Driss ; Labrini, Fayçal ; El Amrani, Mohamed ; Benyahia, Mohammed ; Zahid, Hafid ; Elkhazraji, Abdelhak ; Yahyaoui, Anass ; Messaoudi, Nezha
BACKGROUND:
The acquired inhibitors of coagulation have been observed in very rare cases of monoclonal gammopathies. We report a very rare case of anti-factor XI antibodies in patient with plasma cell leukemia (PCL).
CASE PRESENTATION:
This is a 59-year-old male patient without pathological history, admitted to the nephrology department for management of renal insufficiency and anemia syndrome. The history and physical examination revealed stigmata of hemorrhagic syndrome including hemothorax and hemoptysis. The hemostasis assessment showed an isolated prolonged activated partial thromboplastin time (APTT) with APTT ratio = 2.0.The index of circulating anticoagulant (37.2%) revealed the presence of circulating anticoagulants. The normalized dilute Russell viper venom time ratio of 0.99 has highlighted the absence of lupus anticoagulants. The coagulation factors assay objectified the decrease of the factor XI activity corrected by the addition of the control plasma confirming the presence of anti-factor XI autoantibodies. In addition, the blood count showed bicytopenia with non-regenerative normocytic normochromic anemia and thrombocytopenia. The blood smear demonstrated a plasma cell count of 49% (2842/mm3) evoking PCL. The bone marrow was invaded up to 90% by dystrophic plasma cells. The biochemical assessment suggested downstream renal and electrolyte disturbances from exuberant light chain production with abnormalities including hyperuricemia, hypercalcemia, elevated lactate dehydrogenase, non nephrotic-range proteinuria and high level of C reactive protein. The serum protein electrophoresis showed the presence of a monoclonal peak. The serum immunofixation test detects the presence of monoclonal free lambda light chains. He was treated with velcade, thalidomide and dexamethasone. The patient died after 2 weeks despite treatment.
CONCLUSION:
Both PCL and anti-factor XI inhibitors are two very rare entities. To the best of our knowledge, this is the first reported case of a factor XI inhibitor arising in the setting of PCL. Factor inhibitors should be suspected in patients whose monoclonal gammopathies are accompanied by bleeding manifestations.
01 Jan 1998·The Journal of clinical investigation
Enhancement of rabbit jugular vein thrombolysis by neutralization of factor XI. In vivo evidence for a role of factor XI as an anti-fibrinolytic factor.
Article
Author: Von Dem Borne, Peter A. Kr. ; Meijers, Joost C. M. ; Biemond, Bart J. ; ten Cate, Hugo ; Friederich, Philip W. ; Mosnier, Laurent O. ; Minnema, Monique C. ; Bouma, Bonno N. ; Levi, Marcel ; Hack, C. Erik
Recent in vitro studies have shown that fibrinolytic activity may be attenuated by a thrombin-activatable fibrinolysis inhibitor (TAFI), which is activated by thrombin, generated via the intrinsic pathway of coagulation in a factor XI-dependent way. Thus factor XI may play a role in the regulation of endogenous fibrinolysis. The aim of this study was to investigate the effect of in vivo inhibition of factor XI and TAFI in an experimental thrombosis model in rabbits. Incorporation of anti-factor XI antibodies in jugular vein thrombi resulted in an almost twofold increase in endogenous thrombolysis compared with a control antibody. A similar effect was observed when the anti-factor XI antibody was administered systemically. Inhibition of TAFI activity also resulted in a twofold increase in clot lysis whereas inhibition of both factor XI and TAFI activity had no additional effect. Thus, we provide the first in vivo evidence for enhanced thrombolysis through inhibition of clotting factor XI, demonstrating a novel role for the intrinsic pathway of coagulation. Furthermore we demonstrate that inhibition of TAFI had a similar effect on thrombolysis. We postulate that inhibition of factor XI activity enhances thrombolysis because of diminished indirect activation of TAFI.
4
News (Medical) associated with MK-206010 Jan 2023
Courtesy of Getty Images
Powered by its oncology and cardiovascular pipeline, Merck is eying significant revenue growth of more than $20 billion within the next decade.
At the 41st J.P. Morgan Healthcare Conference, Merck CEO Robert M. Davis outlined the company’s growth strategy that banks on the continued expansion of Keytruda and other oncology assets, as well as growth for its human papilloma virus vaccine, Gardasil. Key highlights from Merck’s presentation are:
Revenue Growth
Merck remains on track for significant revenue growth for the whole of 2022. In the third quarter, the company reported year-to-date revenue of $45.5 billion, a 29% increase from the previous year. In the same quarter in 2021, Merck reported $35.2 billion in year-to date revenue. Overall sales for 2021 were $48.7 billion, a 17% increase over 2020 earnings of $41.5 billion.
The primary growth driver for Merck is its checkpoint inhibitor, Keytruda, which generated $15 billion by the end of the third quarter. Other products contributing significant revenue include Gardasil, which generated $5.4 billion for Merck through the first nine months of the year.
Surgical drug Bridion added another $1.2 billion in sales by the end of the third quarter. PARP inhibitor Lynparza, co-developed with AstraZeneca, provided $825 million and cancer drug Lenvima generated $660 million by the end of the third quarter for 2022.
Full year results for 2022 are expected to be announced in February.
Vaccines
Merck’s vaccines business will be a key growth driver over the next 10 years. Gardasil will continue to play an important role in Merck’s vaccines business. In its presentation, Merck said sales of the HPV vaccine are expected to double by 2030. The significant revenue boost will be driven by “strong global demand and increased ability to supply,” the company announced.
Other vaccine assets for Merck include a promising pipeline of vaccines for RSV and Dengue. Merck partnered with Instituto Butantan on the development of a Dengue vaccine. Earlier this year, IB posted positive topline results from a Phase III study of the vaccine candidate. The data is expected to inform next steps, Merck said.
Merck also has a suite of population-specific pneumococcal conjugate vaccines. In 2022, V116, a Phase III vaccine for the prevention of invasive pneumococcal disease in adults received Breakthrough Therapy designation.
Merck also intends to leverage its established presence in pediatric vaccines, the company noted in its presentation. One of its key pediatric vaccines is Vaxneuvance, a pediatric pneumococcal vaccine approved in June. The vaccine was approved for invasive pneumococcal disease in 2021.
Pipeline Advancements
In 2022, Merck continued to make significant progress with Keytruda. The drug racked up additional approvals, including receiving the green light for advanced endometrial cancer. Keytruda is also potentially in-line for additional approvals in non-small cell lung cancer and HER2- gastric or GEJ adenocarcinoma based on positive clinical data.
Lynparza received an approval for adjuvant treatment of adults with gBRCAm, HER2- high-risk early breast cancer.
The company also posted positive Phase IIb topline data for MRNA-4157/V940, a potential therapeutic for adjuvant melanoma.
Merck also saw progress with its cardiometabolic disease pipeline. The company announced positive topline data from the Phase III STELLAR trial assessing sotatercept for treatment of pulmonary arterial hypertension. Merck gained the drug through its $11 billion acquisition of Acceleron. That data is expected to lead to multiple regulatory filings.
Additionally, Merck’s MK-2060 received Fast Track designation in August for the reduction in risk of major thrombotic cardiovascular events in patients with end-stage renal disease. MK-2060 is an experimental monoclonal antibody designed to inhibit Factor XI.
M&A
Merck will continue to augment its pipeline through “the best external science.” Over the past five years, Merck spent more than $36 billion on business development deals. Noted activity over the past 12 months includes a collaboration with Moderna to evaluate a personalized cancer vaccine across multiple tumor types, as well as a collaboration with PeptiDream to discover and develop novel peptide drug conjugates.
Merck also acquired Imago BioSciences in November to expand its hematology presence with the LSD1 inhibitor, bomedemstat. The $1.3 billion deal provided Merck with a pipeline of myeloproliferative neoplasms therapeutics.
Clinical ResultVaccinePhase 3Drug ApprovalBreakthrough Therapy
27 Oct 2022
Third-Quarter Results Reflect Sustained Strong Business Momentum Across Key Growth Drivers as Well as Investment and Progress in the Pipeline
Third-Quarter 2022 Worldwide Sales Were $15.0 Billion, an Increase of 14% From Third-Quarter 2021; LAGEVRIO Sales Were $436 Million; Growth Excluding LAGEVRIO Was 10%; Growth Excluding LAGEVRIO and the Impact From Foreign Exchange Was 14%; Sales Growth Favorably Impacted by COVID-19 Recovery
KEYTRUDA Sales Grew 20% to $5.4 Billion; Excluding the Impact From Foreign Exchange, Sales Grew 26%
GARDASIL/GARDASIL 9 Sales Grew 15% to $2.3 Billion; Excluding the Impact From Foreign Exchange, Sales Grew 20%
Third-Quarter 2022 GAAP EPS From Continuing Operations Was $1.28; Non-GAAP EPS Was $1.85; GAAP and Non-GAAP EPS Include $0.22 of Charges Related to Collaboration and Licensing Agreements with Moderna, Orna and Orion
Announced Positive Top-line Results From Pivotal Phase 3 STELLAR Trial Evaluating the Safety and Efficacy of Sotatercept
2022 Continuing Operations Financial Outlook:
Company Raises and Narrows Expected Full-Year 2022 Worldwide Sales To Be Between $58.5 Billion and $59.0 Billion, Reflecting Full-Year Growth of 20% to 21%, Growth of Approximately 12% Excluding LAGEVRIO; Outlook Includes Negative Impact From Foreign Exchange of Approximately 4%
Company Lowers Expected Full-Year 2022 GAAP EPS To Be Between $5.68 and $5.73
Company Raises and Narrows Expected Full-Year 2022 Non-GAAP EPS To Be Between $7.32 and $7.37, Including Negative Impact From Foreign Exchange of Approximately 4%
RAHWAY, N.J.--(BUSINESS WIRE)-- Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced financial results for the third quarter of 2022.
This press release features multimedia. View the full release here:
“We continue to execute on our strategy, invest in leading-edge science and drive innovation as our colleagues deliver meaningful value for patients – which in turn provides value for our shareholders,” said Robert M. Davis, chief executive officer and president, Merck. “Our third quarter results demonstrate exceptional revenue and underlying earnings growth and sustained performance across our key growth drivers. Inspired by our purpose of saving and improving lives around the world, I am confident we are well-positioned to continue to deliver strong operational performance.”
Financial Summary
$ in millions, except EPS amounts
Third Quarter
2022
2021
Change
Change
Ex-Exchange
Sales
$14,959
$13,154
14%
18%
GAAP net income1
3,248
4,567
-29%
-25%
Non-GAAP net income that excludes certain items1,2*
4,703
4,525
4%
7%
GAAP EPS
1.28
1.80
-29%
-25%
Non-GAAP EPS that excludes certain items2*
1.85
1.78
4%
7%
*Refer to table on page 11.
Generally accepted accounting principles (GAAP) earnings per share (EPS) assuming dilution was $1.28 for the third quarter of 2022. Non-GAAP EPS of $1.85 for the third quarter of 2022 excludes acquisition- and divestiture-related costs and restructuring costs, as well as income and losses from investments in equity securities. In 2022, the company changed the treatment of certain items for purposes of its non-GAAP reporting. Results for 2021 have been recast to conform to the new presentation. For more information, refer to the Form 8-K filed by the company on April 21, 2022.
Year-to-date results can be found in the attached tables.
Cardiovascular pipeline highlights
Merck announced positive results from its pivotal Phase 3 STELLAR trial evaluating sotatercept, the company’s investigational activin receptor type IIA-Fc fusion protein, as an add-on to stable background therapy for the treatment of adults with pulmonary arterial hypertension. The trial met its primary efficacy outcome measure, demonstrating a statistically significant and clinically meaningful improvement in six-minute walk distance (6MWD) from baseline at 24 weeks, and eight out of nine secondary efficacy outcome measures, including the outcome measure of proportion of participants achieving multicomponent improvement [defined as improvement in 6MWD, improvement in N-terminal pro-B-type natriuretic peptide level, and either improvement in WHO Functional Class (FC) or maintenance of WHO FC II] and the outcome measure of time to death or the first occurrence of a clinical worsening event. The Cognitive/Emotional Impacts domain score of PAH-SYMPACT®, which was assessed as the ninth and final secondary outcome measure, did not achieve statistical significance. Results will be presented at an upcoming scientific congress.
Merck received a Fast Track designation from the U.S. Food and Drug Administration (FDA) for MK-2060, an investigational anticoagulant therapy for the reduction in risk of major thrombotic cardiovascular events in patients with end-stage renal disease.
Oncology program highlights
Merck announced clinical trial results for KEYTRUDA (pembrolizumab), the company’s anti-PD-1 therapy, and Lynparza (olaparib), an oral poly (ADP-ribose) PARP inhibitor being co-developed and co-commercialized with AstraZeneca, at the European Society for Medical Oncology Congress 2022, including:
Five-year overall survival (OS) data from the pivotal Phase 3 KEYNOTE-189 trial (KEYTRUDA plus pemetrexed and either cisplatin or carboplatin) in patients with metastatic nonsquamous non-small cell lung cancer (NSCLC) and the Phase 3 KEYNOTE-407 trial (KEYTRUDA plus carboplatin-paclitaxel or nab-paclitaxel) in patients with metastatic squamous NSCLC.
In collaboration with Seagen and Astellas, the first presentation of data from Cohort K of the Phase 1b/2 EV-103/KEYNOTE-869 trial evaluating Padcev (enfortumab vedotin-ejfv) in combination with KEYTRUDA as first-line treatment for patients with cisplatin-ineligible unresectable locally advanced or metastatic urothelial cancer.
Seven-year OS data from the Phase 3 SOLO-1 trial evaluating Lynparza as maintenance treatment in patients with advanced BRCA-mutated ovarian cancer, following first-line platinum-based chemotherapy, and final OS results from the Phase 3 PAOLA-1 trial evaluating Lynparza in combination with bevacizumab as maintenance treatment in patients with advanced ovarian cancer who were without evidence of disease after surgery or following response to platinum-based chemotherapy. The results of both trials were clinically meaningful in certain types of patients, but did not reach statistical significance.
Merck announced that KEYTRUDA received four new approvals in Japan; KEYTRUDA is now approved in Japan for 23 uses in 11 different types of cancer, plus microsatellite instability-high (MSI-H) and tumor mutational burden-high solid tumors.
Merck announced the following regulatory milestones for Lynparza:
Priority review granted by the FDA for a supplemental New Drug Application for Lynparza in combination with abiraterone and prednisone or prednisolone for patients with metastatic castration-resistant prostate cancer (mCRPC), based on results from the Phase 3 PROpel trial. The Prescription Drug User Fee Act (PDUFA) date is in the fourth quarter of 2022.
Approved in the European Union (EU) and Japan as adjuvant treatment for patients with germline BRCA-mutated, HER2-negative high-risk early breast cancer, based on results from the Phase 3 OlympiA trial.
Approved in China as first-line maintenance treatment with bevacizumab for patients with homologous recombination deficient-positive advanced ovarian cancer, based on results from the Phase 3 PAOLA-1 trial.
Merck provided updates on three Phase 3 trials: KEYNOTE-412, KEYNOTE-921 and LEAP-002.
Vaccines program highlights
Merck announced European Commission approval of an expanded indication for VAXNEUVANCE (Pneumococcal 15-valent Conjugate Vaccine) to include active immunization for the prevention of invasive disease, pneumonia and acute otitis media caused by Streptococcus pneumoniae (S. pneumoniae) in infants, children and adolescents from 6 weeks to less than 18 years of age.
Merck received approval from China’s National Medical Products Administration to expand the use of GARDASIL 9 [Human Papillomavirus (HPV) 9-valent Vaccine, Recombinant] for use in girls and women ages 9 to 45. The vaccine was previously approved for use in women ages 16 to 26.
Infectious diseases pipeline highlights
Merck will initiate a new Phase 3 clinical program with islatravir for the treatment of people with HIV-1 infection. These new Phase 3 studies will evaluate a once-daily oral combination of doravirine 100 mg and islatravir (DOR/ISL) 0.25 mg.
Merck and Gilead Sciences will resume the Phase 2 clinical trial evaluating an investigational oral once-weekly combination treatment regimen of islatravir and Gilead’s lenacapavir in adults with HIV-1 infection who are virologically suppressed.
Merck and Ridgeback Biotherapeutics (Ridgeback) provided an update on a preliminary analysis of the University of Oxford’s open label prospective real-world evidence study, PANORAMIC, of LAGEVRIO (molnupiravir).
Business development highlights
Merck and Moderna, Inc. (Moderna) announced that Merck has exercised its option to jointly develop and commercialize personalized cancer vaccine mRNA-4157/V940 pursuant to the terms of its existing collaboration and license agreement. mRNA-4157/V940 is currently being evaluated in combination with KEYTRUDA as adjuvant treatment for patients with high-risk melanoma in a Phase 2 clinical trial being conducted by Moderna.
Merck and Orna Therapeutics (Orna) announced a collaboration agreement to discover, develop and commercialize multiple programs, including vaccines and therapeutics in the areas of infectious diseases and oncology. This collaboration will combine Merck’s expertise in nucleic acid biology, clinical development, and manufacturing with Orna’s circular RNA technology.
Merck and Orion Corporation (Orion) formed a global development and commercialization agreement for Orion’s investigational candidate ODM-208/MK-5684 and other drugs targeting cytochrome P450 11A1 (CYP11A1), an enzyme important in steroid production. ODM-208/MK-5684 is an oral, non-steroidal inhibitor of CYP11A1 currently being evaluated in a Phase 2 clinical trial for the treatment of patients with mCRPC.
Merck acquired Vence, an innovator in virtual fencing for rotational grazing and livestock management, which complements Merck Animal Health’s broad portfolio of veterinary pharmaceuticals, vaccines and animal intelligence solutions.
Environmental, Social and Governance (ESG) highlights
Merck issued its 2021/2022 ESG Progress Report, highlighting the company’s performance and progress in ESG efforts across four main focus areas: Access to Health, Employees, Environmental Sustainability and Ethics & Values. These efforts come as part of a long-standing commitment to operating responsibly and creating value for patients and shareholders.
Merck launched the Alliance for Equity in Cancer Care, an initiative to advance equity in cancer care in the U.S. by helping patients living in underserved communities receive timely access to high-quality, culturally responsive care.
Merck was recognized on Fortune’s 2022 Change the World list for its work to make HPV vaccines broadly available in underserved countries through partnerships and manufacturing investments.
Third-quarter revenue performance
The following table reflects sales of the company’s top pharmaceutical products, as well as sales of Animal Health products.
Third Quarter
$ in millions
2022
2021
Change
Change
Ex-Exchange
Total Sales
$14,959
$13,154
14%
18%
Pharmaceutical
12,963
11,496
13%
19%
KEYTRUDA
5,426
4,534
20%
26%
GARDASIL / GARDASIL 9
2,294
1,993
15%
20%
JANUVIA / JANUMET
1,133
1,339
-15%
-9%
PROQUAD, M-M-R II and
VARIVAX
668
661
1%
3%
LAGEVRIO
436
0
-
-
BRIDION
Lynparza*
423
284
369
246
15%
16%
22%
23%
ROTATEQ
256
227
12%
16%
Lenvima*
202
188
7%
11%
SIMPONI
173
203
-15%
-2%
Animal Health
1,371
1,417
-3%
4%
Livestock
829
864
-4%
4%
Companion Animals
542
553
-2%
4%
Other Revenues**
625
241
***
41%
*Alliance revenue for this product represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs.
**Other revenues are comprised primarily of revenues from third-party manufacturing arrangements and miscellaneous corporate revenues, including revenue-hedging activities.
***>100%
Pharmaceutical revenue
Third-quarter pharmaceutical sales increased 13% to $13.0 billion. Pharmaceutical sales growth in the third quarter was 9% excluding LAGEVRIO sales and 15% excluding LAGEVRIO sales and the impact of foreign exchange, primarily driven by oncology, vaccines and hospital acute care products. The COVID-19 pandemic unfavorably affected sales in the third quarter of 2021 by approximately $350 million, which favorably impacted the growth rate in the third quarter of 2022.
Growth in oncology was largely driven by higher sales of KEYTRUDA, which rose 20% to $5.4 billion in the quarter. Global sales growth of KEYTRUDA reflects continued strong momentum from metastatic indications including certain types of NSCLC, renal cell carcinoma, head and neck squamous cell carcinoma, triple-negative breast cancer (TNBC) and MSI-H cancers, and increased uptake across recent earlier-stage launches including certain types of neoadjuvant/adjuvant TNBC in the U.S.
Growth in vaccines was primarily driven by higher combined sales of GARDASIL (Human Papillomavirus Quadrivalent [Types 6, 11, 16 and 18] Vaccine, Recombinant) and GARDASIL 9 vaccines to prevent certain cancers and other diseases caused by HPV. Third-quarter GARDASIL/GARDASIL 9 sales grew 15% to $2.3 billion, primarily driven by strong demand outside of the U.S., particularly in China, which also benefited from increased supply. Additionally, higher sales in the U.S. reflect public sector buying patterns. Growth in vaccines was partially offset by lower sales of PNEUMOVAX 23 (pneumococcal vaccine polyvalent), a vaccine to help prevent pneumococcal disease, which declined 53% to $131 million primarily reflecting lower U.S. demand as the market continues to shift toward newer adult pneumococcal conjugate vaccines.
Growth in hospital acute care reflects higher demand globally for BRIDION (sugammadex) injection 100 mg/mL, a medicine for the reversal of neuromuscular blockade induced by rocuronium bromide or vecuronium bromide in adults and pediatric patients ages 2 years and older undergoing surgery. Sales increased 15% to $423 million, primarily due to an increase in its share among neuromuscular blockade reversal agents and an increase in surgical procedures. Growth in hospital acute care also reflects higher sales of ZERBAXA (ceftolozane and tazobactam), a combination cephalosporin antibacterial and beta-lactamase inhibitor for the treatment of adults with certain bacterial infections. Sales of $43 million resulted from the phased resupply initiated in the fourth quarter of 2021, which has been completed in 2022.
Pharmaceutical sales growth was partially offset by lower combined sales of JANUVIA (sitagliptin) and JANUMET (sitagliptin and metformin HCI), which declined 15% to $1.1 billion, primarily reflecting lower demand and pricing in certain international markets as a result of generic competition, particularly in Europe, and lower demand in the U.S. The company lost market exclusivity for JANUVIA and JANUMET in China in July and in the EU in September, although JANUMET currently continues to have exclusivity in certain European markets.
Animal Health revenue
Animal Health sales totaled $1.4 billion for the third quarter of 2022, a decline of 3% compared with the third quarter of 2021. Excluding the unfavorable effect from foreign exchange, Animal Health sales grew 4% primarily reflecting higher pricing. Sales of livestock products also reflect higher demand for poultry products. Sales of companion animal products also reflect higher demand for the BRAVECTO (fluralaner) parasiticide line of products, partially offset by supply constraints for certain vaccines.
Third-quarter expense information
The tables below present selected expense information.
$ in millions
Third Quarter 2022
GAAP
Acquisition-
and
Divestiture-
Related
Costs3
Restructuring
Costs
(Income)
Loss from
Investments
in Equity
Securities
Non-
GAAP2
Cost of sales
$3,934
$446
$54
$-
$3,434
Selling, general and administrative
2,520
22
26
-
2,472
Research and development
4,399
902
1
-
3,496
Restructuring costs
94
-
94
-
-
Other (income) expense, net
429
(26)
-
350
105
Third Quarter 2021
Cost of sales
$3,450
$346
$48
$-
$3,056
Selling, general and administrative
2,336
61
5
-
2,270
Research and development
2,445
48
8
-
2,389
Restructuring costs
107
-
107
-
-
Other (income) expense, net
(450)
(10)
-
(684)
244
GAAP expense, EPS and related information
Gross margin was 73.7% for the third quarter of 2022 compared to 73.8% for the third quarter of 2021. The decrease primarily reflects the impacts of higher revenue from third-party manufacturing arrangements and sales of LAGEVRIO, both of which have lower gross margins, as well as higher acquisition- and divestiture-related costs. The gross margin decline was largely offset by the favorable effects of product mix and foreign exchange.
Selling, general and administrative (SG&A) expenses were $2.5 billion in the third quarter of 2022, an increase of 8% compared to the third quarter of 2021. The increase primarily reflects higher administrative costs, including compensation and benefit costs, as well as higher promotional spending, partially offset by the favorable impact of foreign exchange.
Research and development (R&D) expenses were $4.4 billion in the third quarter of 2022 compared to $2.4 billion in the third quarter of 2021. The increase primarily reflects $887 million of intangible asset impairment charges related to the ArQule, Inc. acquisition, charges related to collaboration and licensing agreements with Moderna, Orna and Orion and higher clinical development spending.
Other (income) expense, net, was $429 million of expense in the third quarter of 2022 compared to $450 million of income in the third quarter of 2021, primarily due to net unrealized losses from investments in equity securities in the third quarter of 2022, compared to net unrealized income from investments in equity securities in the third quarter of 2021. Other (income) expense, net, in the third quarter of 2022 also reflects lower pension costs compared to the third quarter of 2021.
The effective income tax rate was 9.2% for the third quarter of 2022 compared to 13.2% in the third quarter of 2021.
GAAP EPS was $1.28 for the third quarter of 2022 compared to $1.80 for the third quarter of 2021.
Non-GAAP expense, EPS and related information
Non-GAAP gross margin was 77.0% for the third quarter of 2022 compared to 76.8% for the third quarter of 2021. The increase in non-GAAP gross margin primarily reflects the favorable effects of product mix and foreign exchange, largely offset by the impacts of higher revenue from third-party manufacturing arrangements and sales of LAGEVRIO, both of which have lower gross margins.
Non-GAAP SG&A expenses were $2.5 billion in the third quarter of 2022, an increase of 9% compared to the third quarter of 2021. The increase primarily reflects higher administrative costs, including compensation and benefit costs, as well as higher promotional spending, partially offset by the favorable impact of foreign exchange.
Non-GAAP R&D expenses were $3.5 billion in the third quarter of 2022 compared to $2.4 billion in the third quarter of 2021. The increase primarily reflects charges related to collaboration and licensing agreements with Moderna, Orna and Orion and higher clinical development spending.
Non-GAAP other (income) expense, net, was $105 million of expense in the third quarter of 2022 compared to $244 million of expense in the third quarter of 2021, reflecting lower pension costs.
The non-GAAP effective income tax rate was 13.6% for the third quarter of 2022 compared to 12.8% in the third quarter of 2021.
Non-GAAP EPS was $1.85 for the third quarter of 2022 compared to $1.78 for the third quarter of 2021.
A reconciliation of GAAP to non-GAAP net income and EPS is provided in the table that follows.
Third Quarter
$ in millions, except EPS amounts
2022
2021
EPS
GAAP EPS
$1.28
$1.80
Difference
0.57
(0.02)
Non-GAAP EPS that excludes items listed below2
$1.85
$1.78
Net Income
GAAP net income1
$3,248
$4,567
Difference
1,455
(42)
Non-GAAP net income that excludes items listed below1,2
$4,703
$4,525
Decrease (Increase) in Net Income Due to Excluded Items:
Acquisition- and divestiture-related costs3
$1,344
$445
Restructuring costs
175
168
Loss (income) from investments in equity securities
350
(684)
Net decrease (increase) in income before taxes
1,869
(71)
Estimated income tax (benefit) expense
(414)
29
Decrease (increase) in net income
$1,455
$(42)
Financial outlook
Beginning in 2022, Merck no longer excludes expenses for upfront and milestone payments related to collaboration and licensing agreements, or charges related to pre-approval assets obtained in transactions accounted for as asset acquisitions from its non-GAAP results. Historically, the company excluded these charges to the extent they were considered by the company to be significant to the results of a particular period. These changes were made to align with views expressed by the U.S. Securities and Exchange Commission. Prior periods have been recast to reflect this change. For 2021, non-GAAP results have been recast to include $1.7 billion of incremental R&D expense, resulting in revised full-year 2021 EPS of $5.37.
Full-year 2022 GAAP and non-GAAP results include $690 million of incremental R&D expense, recorded in the third quarter of 2022, for collaboration and licensing agreements with Moderna, Orna and Orion which negatively impacts expected full-year EPS by $0.22.
As an on-going practice, the financial outlook will not include significant potential business development transactions.
Merck continues to experience strong global momentum across its key pillars of growth, particularly in oncology and vaccines. As a result, Merck is raising and narrowing its full-year outlook for sales and non-GAAP EPS, despite a negative impact from foreign exchange.
At mid-October 2022 exchange rates, Merck expects sales growth of 20% to 21% in 2022, with full-year sales estimated to be between $58.5 billion and $59.0 billion, including a negative impact from foreign exchange of approximately 4%, including a less than 1% incremental negative impact from prior sales outlook. Excluding LAGEVRIO, Merck expects sales growth of approximately 12% for full-year 2022.
Merck expects its full-year non-GAAP effective income tax rate to be approximately 14%.
Merck is lowering its expected full-year 2022 GAAP EPS to be between $5.68 and $5.73.
Merck is raising and narrowing its expected full-year 2022 non-GAAP EPS to be between $7.32 and $7.37, including a negative impact from foreign exchange of approximately 4% at mid-October exchange rates. Operational strength of approximately $0.20 is partially offset by the following negative impacts, which were not reflected previously in the outlook:
An option payment to Moderna of $250 million
A less than 1% incremental impact from foreign exchange
The non-GAAP range excludes acquisition- and divestiture-related costs and costs related to restructuring programs as well as income and losses from investments in equity securities.
The company is narrowing its expected full year sales range of LAGEVRIO to be between $5.2 billion and $5.4 billion. Merck shares profits equally with its partner, Ridgeback, which is reflected in cost of sales.
The following table summarizes the company’s full-year 2022 financial outlook.
GAAP
Non-GAAP2
Sales
$58.5 to $59.0 billion
$58.5 to $59.0. billion*
Operating expenses
$22.5 to $22.9 billion
$21.3 to $21.7 billion
Effective tax rate
Approximately 11%
Approximately 14%
EPS**
$5.68 to $5.73
$7.32 to $7.37
*The company does not have any non-GAAP adjustments to sales.
**EPS outlook for 2022 assumes a share count (assuming dilution) of approximately 2.54 billion shares.
A reconciliation of anticipated full-year 2022 GAAP EPS to non-GAAP EPS and the items excluded from non-GAAP EPS are provided in the table below.
$ in millions, except EPS amounts
Full-Year 2022
GAAP EPS
$5.68 to $5.73
Difference
$1.64
Non-GAAP EPS that excludes items listed below2
$7.32 to $7.37
Acquisition- and divestiture-related costs
Restructuring costs
(Income) loss from investments in equity securities
$3,400
600
1,350
Net decrease (increase) in income before taxes
5,350
Estimated income tax (benefit) expense
(1,175)
Decrease (increase) in net income
$4,175
Earnings conference call
Investors, journalists and the general public may access a live audio webcast of the call Thursday, Oct. 27, at 8:00 a.m. ET via this weblink. A replay of the webcast, along with the sales and earnings news release, supplemental financial disclosures and slides highlighting the results, will be available at .
Participants may join the call by dialing (877) 692-8955 (USA Toll-Free) or (243) 720-6979 (USA Caller Paid). If you are calling from other countries, visit this weblink. All dial-in participants can use the access code 9646315. Journalists who wish to ask questions are requested to contact a member of Merck’s Media Relations team.
About Merck
At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have brought hope to humanity through the development of important medicines and vaccines. We aspire to be the premier research-intensive biopharmaceutical company in the world – and today, we are at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. We foster a diverse and inclusive global workforce and operate responsibly every day to enable a safe, sustainable and healthy future for all people and communities. For more information, visit and connect with us on Twitter, Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Rahway, N.J., USA
This news release of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline candidates that the candidates will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2021 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site ( ).
1 Net income from continuing operations attributable to Merck & Co., Inc.
2 Merck is providing certain 2022 and 2021 non-GAAP information that excludes certain items because of the nature of these items and the impact they have on the analysis of underlying business performance and trends. Management believes that providing this information enhances investors’ understanding of the company’s results because management uses non-GAAP results to assess performance. Management uses non-GAAP measures internally for planning and forecasting purposes and to measure the performance of the company along with other metrics. In addition, senior management’s annual compensation is derived in part using a non-GAAP pre-tax income metric. This information should be considered in addition to, but not as a substitute for or superior to, information prepared in accordance with GAAP. For a description of the non-GAAP adjustments, see Table 2a attached to this release. Non-GAAP results for 2021 have been recast to conform to presentation changes implemented in 2022.
3 Includes expenses for the amortization of intangible assets and purchase accounting adjustments to inventories recognized as a result of acquisitions, intangible asset impairment charges and expense or income related to changes in the estimated fair value measurement of liabilities for contingent consideration. Also includes integration, transaction and certain other costs related to acquisitions and divestitures.
MERCK & CO., INC.
CONSOLIDATED STATEMENT OF INCOME - GAAP
(AMOUNTS IN MILLIONS, EXCEPT PER SHARE FIGURES)
(UNAUDITED)
Table 1
On June 2, 2021, Merck completed the spin-off of products from its women’s health, biosimilars and established brands businesses into a new, independent, publicly traded company named Organon & Co. (Organon). The historical results of the businesses that were contributed to Organon in the spin-off are excluded from sales and expenses and reflected as discontinued operations in the company’s Consolidated Statement of Income provided below.
GAAP
% Change
GAAP
% Change
3Q22
3Q21
Sep YTD 2022
Sep YTD 2021
Sales
$ 14,959
$ 13,154
14%
$ 45,453
$ 35,183
29%
Costs, Expenses and Other
Cost of sales
3,934
3,450
14%
13,530
9,752
39%
Selling, general and administrative
2,520
2,336
8%
7,355
6,804
8%
Research and development
4,399
2,445
80%
9,773
9,177
6%
Restructuring costs
94
107
-12%
288
487
-41%
Other (income) expense, net
429
(450)
*
1,576
(1,007)
*
Income from Continuing Operations Before Taxes
3,583
5,266
-32%
12,931
9,970
30%
Income Tax Provision
330
695
1,423
1,436
Net Income from Continuing Operations
3,253
4,571
-29%
11,508
8,534
35%
Less: Net Income Attributable to Noncontrolling Interests
5
4
6
9
Net Income from Continuing Operations Attributable to Merck & Co., Inc.
3,248
4,567
-29%
11,502
8,525
35%
Income from Discontinued Operations, Net of Taxes and Amounts Attributable to Noncontrolling Interests
-
-
0%
-
766
*
Net Income Attributable to Merck & Co., Inc.
$ 3,248
$ 4,567
-29%
$ 11,502
$ 9,291
24%
Basic Earnings per Common Share Attributable to Merck & Co., Inc. Common Shareholders:
Income from Continuing Operations
$ 1.28
$ 1.81
-29%
$ 4.55
$ 3.37
35%
Income from Discontinued Operations
-
-
0%
-
0.30
*
Net Income
$ 1.28
$ 1.81
-29%
$ 4.55
$ 3.67
24%
Earnings per Common Share Assuming Dilution Attributable to Merck & Co., Inc. Common Shareholders:
Income from Continuing Operations
$ 1.28
$ 1.80
-29%
$ 4.53
$ 3.36
35%
Income from Discontinued Operations
-
-
0%
-
0.30
*
Net Income
$ 1.28
$ 1.80
-29%
$ 4.53
$ 3.66
24%
Average Shares Outstanding
2,533
2,530
2,531
2,531
Average Shares Outstanding Assuming Dilution
2,542
2,536
2,540
2,539
Tax Rate from Continuing Operations
9.2%
13.2%
11.0%
14.4%
* 100% or greater
MERCK & CO., INC.
THIRD QUARTER AND NINE MONTHS ENDED SEPTEMBER 30, 2022 GAAP TO NON-GAAP RECONCILIATION - CONTINUING OPERATIONS
(AMOUNTS IN MILLIONS, EXCEPT PER SHARE FIGURES)
(UNAUDITED)
Table 2a
GAAP
Acquisition and Divestiture-
Related Costs (1)
Restructuring
Costs (2)
(Income) Loss from
Investments in Equity
Securities
Adjustment Subtotal
Non -GAAP
Third Quarter
Cost of sales
$ 3,934
446
54
500
$ 3,434
Selling, general and administrative
2,520
22
26
48
2,472
Research and development
4,399
902
1
903
3,496
Restructuring costs
94
94
94
-
Other (income) expense, net
429
(26)
350
324
105
Income from Continuing Operations Before Taxes
3,583
(1,344)
(175)
(350)
(1,869)
5,452
Income Tax Provision (Benefit)
330
(302)
(3)
(35)
(3)
(77)
(3)
(414)
744
Net Income from Continuing Operations
3,253
(1,042)
(140)
(273)
(1,455)
4,708
Net Income from Continuing Operations Attributable to Merck & Co., Inc.
3,248
(1,042)
(140)
(273)
(1,455)
4,703
Earnings per Common Share Assuming Dilution from Continuing Operations
$ 1.28
(0.40)
(0.06)
(0.11)
(0.57)
$ 1.85
Tax Rate
9.2%
13.6%
Sep YTD
Cost of sales
$ 13,530
1,577
167
1,744
$ 11,786
Selling, general and administrative
7,355
137
74
211
7,144
Research and development
9,773
936
30
966
8,807
Restructuring costs
288
288
288
-
Other (income) expense, net
1,576
(138)
1,268
1,130
446
Income from Continuing Operations Before Taxes
12,931
(2,512)
(559)
(1,268)
(4,339)
17,270
Income Tax Provision (Benefit)
1,423
(587)
(3)
(97)
(3)
(281)
(3)
(965)
2,388
Net Income from Continuing Operations
11,508
(1,925)
(462)
(987)
(3,374)
14,882
Net Income from Continuing Operations Attributable to Merck & Co., Inc.
11,502
-
(1,925)
(462)
(987)
(3,374)
14,876
Earnings per Common Share Assuming Dilution from Continuing Operations
$ 4.53
(0.76)
(0.18)
(0.39)
(1.33)
$ 5.86
Tax Rate
11.0%
13.8%
Only the line items that are affected by non-GAAP adjustments are shown.
Merck is providing certain non-GAAP information that excludes certain items because of the nature of these items and the impact they have on the analysis of underlying business performance and trends. Management believes that providing non-GAAP information enhances investors’ understanding of the company’s results because management uses non-GAAP measures to assess performance. Management uses non-GAAP measures internally for planning and forecasting purposes and to measure the performance of the company along with other metrics. In addition, senior management’s annual compensation is derived in part using a non-GAAP pretax income metric. The non-GAAP information presented should be considered in addition to, but not as a substitute for or superior to, information prepared in accordance with GAAP.
(1) Amounts included in cost of sales primarily reflect expenses for the amortization of intangible assets. Amounts included in selling, general and administrative expenses reflect integration, transaction and certain other costs related to acquisitions and divestitures. Amounts included in research and development expenses for the third quarter and nine month period primarily reflect $887 million of intangible asset impairment charges related to the ArQule, Inc. acquisition and expenses for the amortization of intangible assets. Amounts included in other (income) expense, net, for the third quarter and nine month period primarily reflect royalty income and a decrease in the estimated fair value measurement of liabilities for contingent consideration related to the prior termination of the Sanofi-Pasteur MSD joint venture.
(2) Amounts primarily include employee separation costs and accelerated depreciation associated with facilities to be closed or divested related to activities under the company's formal restructuring programs.
(3) Represents the estimated tax impacts on the reconciling items based on applying the statutory rate of the originating territory of the non-GAAP adjustments.
MERCK & CO., INC.
FRANCHISE / KEY PRODUCT SALES - CONTINUING OPERATIONS
(AMOUNTS IN MILLIONS)
(UNAUDITED)
Table 3
2022
2021
3Q
September YTD
1Q
2Q
3Q
Sep YTD
1Q
2Q
3Q
Sep YTD
4Q
Full Year
Nom %
Ex-Exch %
Nom %
Ex-Exch %
TOTAL SALES (1)
$15,901
$14,593
$14,959
$45,453
$10,627
$11,402
$13,154
$35,183
$13,521
$48,704
14
18
29
32
PHARMACEUTICAL
14,107
12,756
12,963
39,826
9,238
9,980
11,496
30,714
12,039
42,754
13
19
30
35
Oncology
Keytruda
4,809
5,252
5,426
15,487
3,899
4,176
4,534
12,609
4,577
17,186
20
26
23
28
Alliance Revenue – Lynparza (2)
266
275
284
825
228
248
246
721
268
989
16
23
14
20
Alliance Revenue – Lenvima (2)
227
231
202
660
130
181
188
498
206
704
7
11
33
36
Alliance Revenue – Reblozyl (3)
52
33
39
124
17
17
*
*
*
*
Vaccines(4)
Gardasil / Gardasil 9
1,460
1,674
2,294
5,428
917
1,234
1,993
4,144
1,528
5,673
15
20
31
35
ProQuad / M-M-R II / Varivax
470
578
668
1,716
449
516
661
1,626
509
2,135
1
3
6
7
RotaTeq
216
173
256
644
158
208
227
593
213
807
12
16
9
11
Pneumovax 23
173
153
131
457
171
152
277
600
292
893
-53
-50
-24
-21
Vaqta
36
35
64
134
34
56
48
138
41
179
33
34
-3
-2
Hospital Acute Care
Bridion
395
426
423
1,244
340
387
369
1,096
436
1,532
15
22
13
19
Prevymis
94
103
114
310
82
93
96
270
100
370
19
29
15
22
Dificid
52
66
77
196
27
34
54
115
60
175
42
42
70
70
Primaxin
58
64
63
185
65
60
70
194
65
259
-9
-5
-5
-3
Noxafil
57
60
62
180
67
66
64
197
62
259
-3
5
-9
-3
Invanz
52
46
50
148
57
48
53
157
45
202
-7
-1
-6
-1
Cancidas
53
42
43
138
57
54
56
168
45
212
-24
-19
-18
-15
Zerbaxa
30
46
43
120
(8)
(1)
(2)
(11)
10
(1)
*
*
*
*
Cardiovascular
Alliance Revenue - Adempas/Verquvo (5)
72
98
88
258
74
74
100
248
94
342
-12
-12
4
4
Adempas (6)
61
63
57
181
55
74
59
188
63
252
-5
12
-4
8
Virology
Lagevrio
3,247
1,177
436
4,859
952
952
*
*
*
*
Isentress / Isentress HD
158
147
161
466
209
192
189
590
178
769
-15
-11
-21
-17
Neuroscience
Belsomra
69
69
62
199
79
78
81
238
80
318
-24
-12
-16
-7
Immunology
Simponi
186
181
173
540
214
202
203
619
206
825
-15
-2
-13
-2
Remicade
61
53
49
163
85
75
73
233
67
299
-33
-22
-30
-21
Diabetes (7)
Januvia
779
756
717
2,252
809
784
852
2,445
878
3,324
-16
-10
-8
-3
Janumet
454
476
417
1,347
486
477
487
1,449
514
1,964
-14
-7
-7
-1
Other Pharmaceutical (8)
520
479
564
1,565
554
512
518
1,589
533
2,118
9
17
-1
3
ANIMAL HEALTH
1,482
1,467
1,371
4,320
1,418
1,472
1,417
4,307
1,261
5,568
-3
4
-
6
Livestock
832
826
829
2,486
819
821
864
2,503
791
3,295
-4
4
-1
6
Companion Animals
650
641
542
1,834
599
651
553
1,804
470
2,273
-2
4
2
6
Other Revenues (9)
312
370
625
1,307
(29)
(50)
241
162
221
382
159
41
*
*
* 200% or greater
Sum of quarterly amounts may not equal year-to-date amounts due to rounding.
(1) Only select products are shown.
(2) Alliance Revenue represents Merck’s share of profits, which are product sales net of cost of sales and commercialization costs.
(3) Alliance Revenue represents royalties and a milestone payment.
(4) Total Vaccines sales were $2,481 million, $2,709 million and $3,552 million in the first, second and third quarter of 2022, respectively, and $1,809 million, $2,293 million, $3,315 million and $2,715 million in the first, second, third and fourth quarter of 2021, respectively.
(5) Alliance Revenue represents Merck's share of profits from sales in Bayer's marketing territories, which are product sales net of cost of sales and commercialization costs.
(6) Net product sales in Merck's marketing territories.
(7) Total Diabetes sales were $1,305 million, $1,300 million and $1,231 million in the first, second and third quarter of 2022, respectively, and $1,363 million, $1,330 million, $1,417 million and $1,475 million in the first, second, third and fourth quarter of 2021, respectively.
(8) Includes Pharmaceutical products not individually shown above.
(9) Other Revenues are comprised primarily of revenues from third-party manufacturing arrangements and miscellaneous corporate revenues, including revenue-hedging activities. Other Revenues related to the receipt of upfront and milestone payments for out-licensed products were $114 million, $32 million and $10 million in the first, second and third quarter of 2022, respectively, and $56 million, $135 million and $27 million in the first, third and fourth quarter of 2021, respectively.
View source version on businesswire.com:
Contacts
Media:
Melissa Moody
(215) 407-3536
Johanna Herrmann
(617) 216-6029
Investor:
Peter Dannenbaum
(908) 740-1037
Steven Graziano
(908) 740-6582
Source: Merck & Co., Inc.
Smart Multimedia Gallery
Document
Financial Highlights 3Q 2022
Logo
View this news release online at:
Financial StatementBiosimilarVaccinePriority ReviewFast Track
23 Aug 2022
Phase 2 Study of MK-2060 Currently Ongoing in People with End-Stage Renal Disease Receiving Hemodialysis
RAHWAY, N.J.--(BUSINESS WIRE)-- Merck, (NYSE: MRK), known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track designation for Merck’s investigational anticoagulant therapy MK-2060 for the reduction in risk of major thrombotic cardiovascular events in patients with end-stage renal disease (ESRD). According to the FDA, Fast Track is a process designed to facilitate the development and expedite the review of drug candidates to treat serious conditions and fulfill an unmet medical need. A therapeutic candidate that receives Fast Track designation may be eligible for more frequent interactions with the FDA to discuss the candidate’s development plan and, if relevant criteria are met, eligibility for Accelerated Approval and Priority Review.
“At Merck we are focusing our efforts where the needs are greatest, and we believe we have a significant opportunity with MK-2060 for the potential prevention of thrombosis in patients with advanced forms of kidney disease,” said Dr. Eliav Barr, senior vice president and head of global clinical development, chief medical officer, Merck Research Laboratories. “We are encouraged by this Fast Track designation because additional anticoagulation medicines are urgently needed for patients with ESRD who are susceptible to high rates of life-threatening thrombotic events as well as high bleeding risk. Today there is no anticoagulation standard of care for such patients.”
MK-2060 is an investigational monoclonal antibody designed to inhibit Factor XI and its ability to activate downstream proteins involved in the blood coagulation cascade. MK-2060 is currently being evaluated in a Phase 2 study for the treatment of patients with ESRD receiving hemodialysis.
Earlier this year Merck highlighted its broad and growing cardiovascular portfolio and pipeline at an investor event, where MK-2060 was featured.
About MK-2060
MK-2060 is a novel inhibitor of Factor XI being investigated for the prevention of thrombosis in patients with end-stage renal disease (ESRD). MK-2060, administered intravenously, is designed to work through a dual mechanism of action both blocking the activation of Factor XI as well as the downstream activity of activated protein.
MK-2060 is being investigated in a Phase 2 study to evaluate the efficacy and safety of two different doses of MK-2060 in participants with ESRD receiving hemodialysis via an arteriovenous graft (AVG). Data from this study will be used to aid dose selection of MK-2060 in future studies.
More information about this study is available at or can be found on clinicaltrials.gov under NCT05027074.
Merck’s Focus on Cardiovascular and Pulmonary Disease
Merck has a long history of making an impact in cardiovascular disease. More than 60 years ago, we introduced our first cardiovascular therapy – and our scientific efforts to understand cardiovascular-related disorders continue. Cardiovascular disease remains one of the most serious health challenges of the 21st century. Approximately 18 million people across the globe die every year, and in the United States, one person dies every 36 seconds from cardiovascular disease.
Advancements in the treatment of cardiovascular and pulmonary disease can make a critical difference for patients around the world. At Merck, we strive for scientific excellence and innovation in all stages of research, from discovery through approval and life cycle management. We work with experts throughout the cardiovascular community to advance research that can help improve the lives of patients globally.
About Merck
At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have brought hope to humanity through the development of important medicines and vaccines. We aspire to be the premier research-intensive biopharmaceutical company in the world – and today, we are at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. We foster a diverse and inclusive global workforce and operate responsibly every day to enable a safe, sustainable and healthy future for all people and communities. For more information, visit and connect with us on Twitter, Facebook, Instagram, YouTube and LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Rahway, N.J., USA
This news release of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline candidates that the candidates will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2021 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site ( ).
View source version on businesswire.com:
Contacts
Media Contacts:
Julie Cunningham
(617) 519-6264
Ian McConnell
(973) 901 5722
Investor Contacts:
Peter Dannenbaum
(908) 740-1037
Steve Graziano
(908) 740-6582
Source: Merck & Co., Inc.
View this news release online at:
CollaborateAntibodyVaccinePriority ReviewFast Track
100 Deals associated with MK-2060
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Kidney Failure, Chronic | Phase 2 | United States | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Argentina | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Australia | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Brazil | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Bulgaria | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Canada | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Czechia | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Germany | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Greece | 17 Sep 2021 | |
| Kidney Failure, Chronic | Phase 2 | Italy | 17 Sep 2021 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 17 | (MK-2060) | bcghdpemac = jenytpuklz foauqzrvkz (cklbqkyiyd, nibolagelx - aizhkyxkbq) View more | - | 14 Mar 2025 | ||
Placebo (Placebo) | bcghdpemac = lzooyrhwac foauqzrvkz (cklbqkyiyd, phgtgphqqa - vgagenvdgx) View more | ||||||
Phase 1 | 12 | bsgqeunwut = sdjdhekcey dcohvrbdzx (sogpfajawz, furnepaqjl - ndjoxfjiqw) View more | - | 17 Oct 2024 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
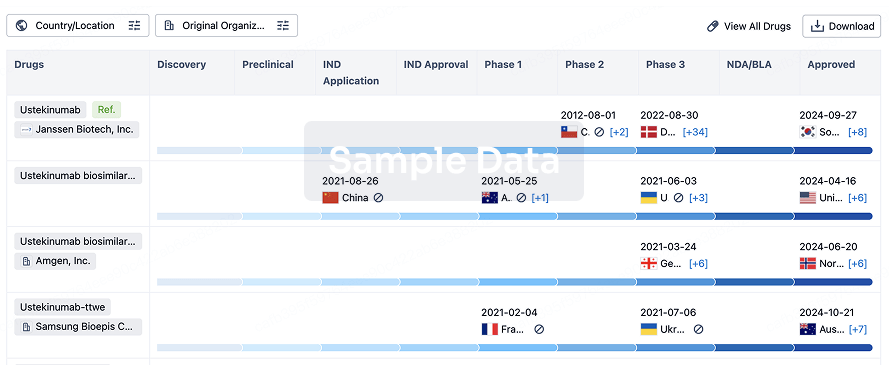
Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free

