Request Demo
Last update 16 May 2025
ACT-709478
Last update 16 May 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms Apinocaltamide, ACT 709478, NBI 827104 + [1] |
Target |
Action blockers |
Mechanism T-type calcium channel blockers(Voltage-gated T-type calcium channel blockers) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseDiscontinuedPhase 2 |
First Approval Date- |
RegulationOrphan Drug (United States) |
Login to view timeline
Structure/Sequence
Molecular FormulaC22H18F3N5O |
InChIKeyLSYANGLAZUZYFX-UHFFFAOYSA-N |
CAS Registry1838651-58-3 |
Related
7
Clinical Trials associated with ACT-709478NCT05301894
Long-Term, Open-Label Extension Study to Evaluate the Safety and Tolerability of NBI-827104 in Pediatric Subjects With Epileptic Encephalopathy With Continuous Spike-and-Wave During Sleep
The primary objective for this study is to evaluate the long-term safety and tolerability of NBI-827104 in pediatric participants with epileptic encephalopathy with continuous spike-and-wave during sleep (EECSWS).
Start Date07 Jun 2022 |
Sponsor / Collaborator |
NCT04625101
Phase 2 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Assess the Efficacy, Safety, Tolerability, and Pharmacokinetics of NBI-827104 in Pediatric Subjects With Epileptic Encephalopathy With Continuous Spike-and-Wave During Sleep
This is a phase 2, double-blind study to assess the efficacy, safety, tolerability, and pharmacokinetics of NBI-827104 when administered once daily for 13 weeks in pediatric subjects with Epileptic Encephalopathy with Continuous Spike-and-Wave During Sleep (EECSWS).
Start Date26 Apr 2021 |
Sponsor / Collaborator |
NCT04880616
A Phase 2 Randomized, Double-Blind, Placebo-Controlled, Crossover Study to Assess the Safety, Tolerability, Pharmacokinetics and Efficacy of NBI-827104 in Subjects With Essential Tremor
The main objective of this study is to evaluate the efficacy, safety, and tolerability of NBI-827104 in adults with essential tremor.
Start Date20 Apr 2021 |
Sponsor / Collaborator |
100 Clinical Results associated with ACT-709478
Login to view more data
100 Translational Medicine associated with ACT-709478
Login to view more data
100 Patents (Medical) associated with ACT-709478
Login to view more data
9
Literatures (Medical) associated with ACT-70947801 Mar 2020·CNS drugsQ2 · MEDICINE
Multiple-Ascending Dose Study in Healthy Subjects to Assess the Pharmacokinetics, Tolerability, and CYP3A4 Interaction Potential of the T-Type Calcium Channel Blocker ACT-709478, A Potential New Antiepileptic Drug
Q2 · MEDICINE
Article
Author: Dingemanse, Jasper ; Ort, Marion ; Kornberger, Rüdiger ; Richard, Muriel ; Kaufmann, Priska
BACKGROUND:
ACT-709478 is a selective, orally available T-type calcium channel blocker being studied as a potential new treatment in epilepsy. ACT-709478 had previously been investigated in a single-ascending dose study up to a dose of 400 mg.
OBJECTIVES:
The aim of this study was to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple doses of ACT-709478. In addition, the drug-drug interaction potential of multiple doses of ACT-709478 with the cytochrome P450 3A4 substrate midazolam was investigated.
METHODS:
This double-blind, placebo-controlled, randomized study included 46 healthy male and female subjects. Ascending multiple oral doses of ACT-709478 were administered to 10 (cohorts 1-2) or 12 (cohorts 3-4) subjects (two taking placebo per cohort). In cohorts 1-2, 30 or 10 mg ACT-709478 was administered once daily for 12 days. An up-titration regimen was used in cohorts 3-4 with administration of 10, 30, and 60 mg for 7 days each in both cohorts and an additional dose level of 100 mg ACT-709478 once daily for 8 days in cohort 4. Single doses of midazolam were administered at baseline and concomitantly to 60 mg and 100 mg ACT-709478 in cohort 4. Blood sampling for pharmacokinetic evaluations and safety assessments (clinical laboratory, vital signs, adverse events, and electrocardiogram) were performed regularly. Holter electrocardiograms were recorded at baseline and for 24 h at steady state and central nervous system effects were assessed with pharmacodynamic tests at baseline and steady state.
RESULTS:
ACT-709478 was absorbed with a time to reach the maximum plasma concentration of 3.5-4.0 h and eliminated with a half-life of 45-53 h. Steady state was reached after 5-7 days of dosing and exposure increased dose-proportionally. An accumulation index of approximately three fold was observed in cohorts 1 and 2. Exposure to midazolam was lower upon concomitant administration of 60 and 100 mg ACT-709478 compared to midazolam alone while the half-life and time to reach the maximum plasma concentration of midazolam remained unchanged, suggesting a weak induction at the gastrointestinal but not hepatic level. Pharmacokinetic parameters of 1-hydroxymidazolam were not affected by ACT-709478 administration. The most frequent adverse events were dizziness, somnolence, and headache. A tolerability signal was detected in cohort 1 (30 mg once daily); therefore, the dose was decreased to 10 mg once daily in cohort 2. The subsequently established up-titration regimen, starting with 10 mg once daily, considerably improved tolerability. Multiple doses up to 100 mg once daily were well tolerated. No treatment-related effects were detected on vital signs, clinical laboratory tests, Holter electrocardiogram variables, or in the pharmacodynamic tests.
CONCLUSIONS:
ACT-709478 exhibits good tolerability up to 100 mg once daily using an up-titration regimen and pharmacokinetic properties that support further clinical investigations. A weak induction of gastrointestinal cytochrome P450 3A4 activity was observed, unlikely to be of clinical relevance. CLINICALTRIALS.
GOV IDENTIFIER:
NCT03165097.
01 May 2019·EpilepsiaQ1 · MEDICINE
First‐in‐man study of ACT ‐709478, a novel selective triple T‐type calcium channel blocker
Q1 · MEDICINE
Article
Author: Dingemanse, Jasper ; Richard, Muriel ; Kornberger, Rüdiger ; Kaufmann, Priska
Summary:
Objective:
Increased activity of T‐type Ca2+ channels is linked to idiopathic generalized epilepsies, thus blocking these channels may be a new treatment option. ACT‐709478 is an orally available triple T‐type Ca2+ channel blocker. The aim of this first‐in‐man study was to investigate the pharmacokinetics, pharmacodynamics, tolerability, and safety of single doses of ACT‐709478 in healthy subjects.
Methods:
This double‐blind, placebo‐controlled, randomized study included 65 healthy male subjects. Ascending single oral doses of 1‐400 mg ACT‐709478 or placebo were administered to sequential groups of eight subjects (6 on active, 2 on placebo). Effect of food was tested in a crossover part at 60 mg. Blood and saliva sampling for pharmacokinetic evaluations and safety assessments was performed regularly. Effects on the central nervous system were assessed with a battery of pharmacodynamic tests.
Results:
The maximum plasma concentration (Cmax) was reached within 3 to 4 hours (≤60 mg) and within 20 to 28 hours (>60 mg), and across all dose levels the terminal half‐life (95% confidence interval) ranged from 36 (29‐45) to 43 (22‐86) hours. Multiple peaks were observed and Cmax and area under the plasma concentration‐time curve (AUC)0‐∞ increased in a less than dose‐proportional manner. A 1.6‐fold increase in Cmax and no change in AUC0‐∞ was observed in fed compared to fasted conditions. A significant correlation (P < 0.0001) between plasma and saliva concentrations was established using linear regression. All adverse events were transient and of mild or moderate intensity. No treatment‐related effects on vital signs, clinical laboratory, telemetry, or electrocardiography were detected. The results of pharmacodynamic tests did not show relevant mean changes compared to baseline or placebo.
Significance:
ACT‐709478 exhibits good tolerability and safety after single‐dose administration and its pharmacokinetic and pharmacodynamic properties warrant further investigations.
02 Dec 2018·Expert opinion on therapeutic patentsQ2 · MEDICINE
T-type calcium channel blockers: a patent review (2012–2018)
Q2 · MEDICINE
Review
Author: Nam, Ghilsoo
INTRODUCTION:
T-type calcium channels are attractive targets for potential treatment of epilepsy inflammatory or neuropathic pain, insomnia, Parkinson's disease, and cancer. Three isoforms having different biophysical functions are expressed in peripheral and central nerve. Since the withdrawal of mibefradil, the first compound marketed for selective T-type calcium channel blockade, extensive efforts have been made to identify more selective T-type calcium channel blockers.
AREAS COVERED:
This review covers the 43 patents describing 'organic small molecules as T-type calcium channel blockers'-published since 2012. The most recent similar patent review was published in 2011. Information from a recent review article and relevant research papers has been included, as well as biological data and clinical trial results where available.
EXPERT OPINION:
Triazinone derivatives, carbazole compounds, and aryl triazole/imidazole amide derivatives display potent blockade activity α1H, α1G, and pan T-type calcium channel subtypes, respectively, though the specificity of the letter is still unsatisfactory. Nonetheless, improvements seen in the efficacy of compounds targeting α1H T-type calcium channels indicate significant progress. Ongoing clinical trials are for the candidates Z944 (Phase II) and ACT-709478 (Phase II) appear promising. These studies may lead to a new generation of inhibitors with higher selectivity, improved physicochemical properties, and reduced side effects.
24
News (Medical) associated with ACT-70947830 Oct 2024
INGREZZA® (valbenazine) Third Quarter Net Product Sales of $613 Million Representing 26% Year-Over-Year Growth
INGREZZA
® (valbenazine) 2024 Net Product Sales Guidance Raised to $2.30 - $2.32 Billion
Board Authorizes $300 Million Share Repurchase Plan
SAN DIEGO, Oct. 30, 2024 /PRNewswire/ -- Neurocrine Biosciences, Inc. (Nasdaq: NBIX) today announced its financial results for the third quarter ended September 30, 2024 and provided an update on its 2024 financial guidance.
"With continued INGREZZA growth across the tardive dyskinesia and Huntington's disease chorea indications, FDA Priority Review for crinecerfont in congenital adrenal hyperplasia, a deep neuroscience focused pipeline and a strong balance sheet, we are confident in our ability to help more patients than ever before," said Kyle W. Gano, Ph.D., Chief Executive Officer of Neurocrine Biosciences.
William Rastetter, Chairman of the Board of Directors of Neurocrine Biosciences, said, "The share repurchase authorization reflects the Board's confidence in Neurocrine's significant value creation potential. Importantly, the new share repurchase authorization preserves our flexibility to drive sustained growth through investments in INGREZZA and the anticipated launch of crinecerfont, while also advancing our diverse pipeline and maintaining our strong balance sheet."
Financial Highlights
INGREZZA Net Product Sales Highlights
INGREZZA third quarter 2024 net product sales were $613 million and grew 26% compared to the third quarter 2023
Year-over-year growth driven by strong underlying patient demand and improvement in gross-to-net dynamics
Other Key Financial Highlights
Differences in third quarter 2024 GAAP and Non-GAAP operating expenses compared with third quarter 2023 were driven by:
Increased R&D expense in support of an expanded and advancing portfolio including investments in muscarinic compounds, gene therapy programs, and second generation VMAT2 inhibitors. Third quarter 2024 R&D expense includes $39 million for development milestones achieved under collaborations with Nxera Pharma UK Limited (Nxera, formerly known as Sosei Heptares) and Voyager Therapeutics, Inc. (Voyager).
Increased SG&A expense includes incremental investment in crinecerfont-related headcount, crinecerfont-related pre-launch activities, and continued investment in INGREZZA, including the recent expansion of our psychiatry and long-term care sales teams in September 2024.
Third quarter 2024 GAAP net income and earnings per share were $130 million and $1.24, respectively, compared with $83 million and $0.82, respectively, for third quarter 2023
Third quarter 2024 Non-GAAP net income and earnings per share were $189 million and $1.81, respectively, compared with $156 million and $1.54, respectively, for third quarter 2023
Differences in third quarter 2024 GAAP and Non-GAAP net income compared with third quarter 2023 driven by:
Higher INGREZZA net sales and improved operating margin
Third quarter 2024 includes $17 million loss from changes in fair values of equity investments compared with $40 million loss for third quarter 2023 (Non-GAAP adjustment)
Third quarter 2024 includes $39 million of expense for development milestones achieved under collaborations with Nxera and Voyager
At September 30, 2024, the Company had cash, cash equivalents and marketable securities totaling approximately $1.9 billion
A reconciliation of GAAP to Non-GAAP financial results can be found in Table 3 and Table 4 at the end of this news release.
Recent Developments
Kyle W. Gano, Ph.D. appointed Chief Executive Officer effective October 11, 2024.
Announced the Company's Board of Directors has authorized a $300 million share repurchase plan. The Company subsequently intends to enter into a $300 million accelerated share repurchase transaction in the coming days, subject to market conditions, which will constitute the entirety of the authorized share repurchase plan.
Announced positive topline data for the Phase 2 study of NBI-1117568, a first-in-class, orally active, highly selective investigational M4 agonist, in development as a potential treatment for schizophrenia. The successful completion of the Phase 2 study triggered a $35 million milestone payment to Nxera in the third quarter of 2024. We expect to advance NBI-1117568 into Phase 3 development in the first half of 2025, which would trigger an additional $15 million milestone payment to Nxera upon initiation of the Phase 3 study.
Presented KINECT
®-HD2 interim data at the 2024 MDS International Congress of Parkinson's Disease and Movement Disorders demonstrating robust and sustained improvements in chorea associated with Huntington's Disease through week 104 irrespective of antipsychotic use.
Announced the ERUDITE™ Phase 2 study of luvadaxistat (NBI-1065844) in cognitive impairment associated with schizophrenia (CIAS) did not meet its primary endpoint. In addition, we provided Takeda Pharmaceutical Company Limited with written notice of termination of the license agreement to develop and commercialize luvadaxistat and NBI-1065846. The termination is anticipated to be effective in April 2025.
Provided Idorsia Pharmaceuticals Ltd. with written notice of termination of the license agreement to develop and commercialize NBI-827104 in epileptic encephalopathy with continuous spike and wave during sleep. The termination is anticipated to be effective in January 2025.
Raised 2024 Net Sales Guidance and Updated Expense Guidance
Conference Call and Webcast Today at 8:00 AM Eastern Time
Neurocrine Biosciences will hold a live conference call and webcast today at 8:00 a.m. Eastern Time (5:00 a.m. Pacific Time). Participants can access the live conference call by dialing 800-225-9448 (US) or 203-518-9708 (International) using the conference ID: NBIX. The webcast and accompanying slides can also be accessed at approximately 8:00 a.m. Eastern Time on Neurocrine Biosciences' website under Investors at . A replay of the webcast will be available on the website approximately one hour after the conclusion of the event and will be archived for approximately one month.
About Neurocrine Biosciences
Neurocrine Biosciences is a neuroscience-focused, biopharmaceutical company with a simple purpose: to relieve suffering for people with great needs, but few options. We are dedicated to discovering and developing life-changing treatments for patients with under-addressed neurological, neuroendocrine, and neuropsychiatric disorders. The company's diverse portfolio includes FDA-approved treatments for tardive dyskinesia, chorea associated with Huntington's disease, endometriosis* and uterine fibroids*, as well as a robust pipeline including multiple compounds in mid- to late-phase clinical development across our core therapeutic areas. For three decades, we have applied our unique insight into neuroscience and the interconnections between brain and body systems to treat complex conditions. We relentlessly pursue medicines to ease the burden of debilitating diseases and disorders, because you deserve brave science. For more information, visit neurocrine.com, and follow the company on LinkedIn, X (Formerly Twitter) and Facebook. (*in collaboration with AbbVie)
NEUROCRINE BIOSCIENCES, NEUROCRINE and YOU DESERVE BRAVE SCIENCE are registered trademarks of Neurocrine Biosciences, Inc. The Neurocrine logo is a trademark of Neurocrine Biosciences, Inc.
Non-GAAP Financial Measures
In addition to the financial results and financial guidance that are provided in accordance with accounting principles generally accepted in the United States (GAAP), this press release also contains the following Non-GAAP financial measures: Non-GAAP R&D expense, Non-GAAP SG&A expense, and Non-GAAP net income and net income per share. When preparing the Non-GAAP financial results and guidance, the Company excludes certain GAAP items that management does not consider to be normal, including recurring cash operating expenses that might not meet the definition of unusual or non-recurring items. In particular, these Non-GAAP financial measures exclude: non-cash stock-based compensation expense, charges associated with convertible senior notes, vacated legacy campus facility costs, net of sublease income, non-cash amortization expense related to acquired intangible assets, acquisition and integration costs, changes in fair value of equity security investments, changes in foreign currency exchange rates and certain adjustments to income tax expense. These Non-GAAP financial measures are provided as a complement to results provided in accordance with GAAP as management believes these Non-GAAP financial measures help indicate underlying trends in the Company's business, are important in comparing current results with prior period results and provide additional information regarding the Company's financial position. Management also uses these Non-GAAP financial measures to establish budgets and operational goals that are communicated internally and externally and to manage the Company's business and evaluate its performance. The Company provides guidance regarding combined R&D and SG&A expenses on both a GAAP and a Non-GAAP basis. A reconciliation of these GAAP financial results to Non-GAAP financial results is included in the attached financial information.
Forward-Looking Statements
In addition to historical facts, this press release contains forward-looking statements that involve a number of risks and uncertainties. These statements include, but are not limited to, statements related to: the benefits to be derived from our products and product candidates; the value our products and/or our product candidates may bring to patients; the continued success of INGREZZA; our financial and operating performance, including our future revenues, expenses, or profits; our collaborative partnerships; expected future clinical and regulatory milestones; the timing of the initiation and/or completion of our clinical, regulatory, and other development activities and those of our collaboration partners; and our intention to enter into an accelerated share repurchase transaction, including the expected dollar amounts and the timing of the transaction. Among the factors that could cause actual results to differ materially from those indicated in the forward-looking statements are: our future financial and operating performance; risks and uncertainties associated with the commercialization of INGREZZA; risks that the crinecerfont New Drug Applications (NDAs) may not obtain regulatory approval, such approval may be delayed, or may not receive the benefits associated with priority review; risks related to the development of our product candidates; risks associated with our dependence on third parties for development, manufacturing, and commercialization activities for our products and product candidates, and our ability to manage these third parties; risks that the FDA or other regulatory authorities may make adverse decisions regarding our products or product candidates; risks that clinical development activities may not be initiated or completed on time or at all, or may be delayed for regulatory, manufacturing, or other reasons, may not be successful or replicate previous clinical trial results, may fail to demonstrate that our product candidates are safe and effective, or may not be predictive of real-world results or of results in subsequent clinical trials; risks that the potential benefits of the agreements with our collaboration partners may never be realized; risks that our products, and/or our product candidates may be precluded from commercialization by the proprietary or regulatory rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; risks associated with government and third-party regulatory and/or policy efforts which may, among other things, impose sales and pharmaceutical pricing controls on our products or limit coverage and/or reimbursement for our products; risks associated with competition from other therapies or products, including potential generic entrants for our products; constraints, volatility, or disruptions in the capital markets or other factors affecting our ability to enter into or complete an accelerated share repurchase transaction; and other risks described in our periodic reports filed with the SEC, including our Quarterly Report on Form 10-Q for the quarter ended September 30, 2024. Neurocrine Biosciences disclaims any obligation to update the statements contained in this press release after the date hereof other than as required by law.
SOURCE Neurocrine Biosciences, Inc.
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED

Phase 2License out/inFinancial StatementExecutive Change
29 Oct 2024
Ad hoc announcement pursuant to Art. 53 LR Allschwil, Switzerland – October 29, 2024 Idorsia Ltd (SIX: IDIA) today announced its financial results for the first nine months of 2024. Highlights Net revenue 9M 2024 at CHF 53 million.US GAAP operating loss 9M 2024 of CHF 154 million and Non-GAAP operating loss of CHF 248 million.Improved Guidance for 2024, driven by diligent cost control.QUVIVIQ™ (daridorexant) total net sales of CHF 39 million in 9M 2024.Commercial partnership for QUVIVIQ with Menarini France.QUVIVIQ approved for the treatment of insomnia in Japan.TRYVIO™ (aprocitentan) commercially available in the US since October 2024.JERAYGO™ (aprocitentan) approved by the European Commission in June 2024 and marketing authorization applications submitted in the UK, Canada, and Switzerland.Collaboration with Neurocrine Biosciences comes to an end. André C. Muller, Chief Executive Officer of Idorsia, commented:“The one thing everyone wants to hear about – both internally and externally – is the status of a deal with aprocitentan. I’m pleased to share that our efforts on this front are advancing well. Our improved financial guidance includes our cost-conscious attitude across the whole organization, without compromising advancements, such as making progress in ramping up sales of QUVIVIQ, making TRYVIO available in the US, and expanding marketing authorization for JERAYGO.” Financial results US GAAP resultsNine MonthsThird Quarterin CHF millions, except EPS (CHF) and number of shares (millions)2024202320242023Net revenues531312680Operating expenses(211)(275)(118)150Operating income (loss)(154)(144)(90)231Net income (loss)(180)(181)(101)224Basic EPS(1.00)(1.02)(0.55)1.26Basic weighted average number of shares180.5178.2182.4178.4Diluted EPS(1.00)(1.02)(0.55)0.96Diluted weighted average number of shares180.5178.2182.4232.5 Net revenue of CHF 53 million in the first nine months of 2024 is the result of QUVIVIQ product sales (CHF 39 million), product sales to partners in the Asia-Pacific-Region (CHF 9 million) and contract revenue recognized in connection with Owkin (CHF 3 million). This compares to CHF 131 million in the first nine months of 2023, which included CHF 34 million sales of PIVLAZ in Japan (assigned in the meantime to Nxera Pharma as part of a transaction, more details can be found in the dedicated press release) and CHF 68 million one-off impact of the Nxera deal as well as CHF 4 million revenue share from Johnson & Johnson related to ponesimod sales (revenue-sharing agreement now eliminated as part of the reacquisition of aprocitentan, more details can be found in the dedicated press release). US GAAP operating expenses in the first nine months of 2024 benefited from extraordinary income of CHF 125 million from the Viatris deal resulting in an expense of CHF 211 million (CHF 275 million in the first nine months of 2023), of which CHF 16 million related to cost of sales (CHF 7 million in the first nine months of 2023), CHF 111 million to R&D expenses (CHF 235 million in the first nine months of 2023), and CHF 209 million to SG&A expenses (CHF 318 million in the first nine months of 2023). US GAAP net loss in the first nine months of 2024 amounted to CHF 180 million (CHF 181 million net loss in the first nine months of 2023). The net loss was favorably impacted by a one-off income related to the Viatris deal of CHF 125 million (CHF 302 million one-off income related to the Nxera deal in the first nine months of 2023) and lower operating expenses throughout all functions. The US GAAP net loss resulted in a basic net loss per share of CHF 1.00 (basic and diluted) in the first nine months of 2024, compared to a net loss per share of CHF 1.02 (basic and diluted) in the first nine months of 2023. Non-GAAP* measuresNine MonthsThird Quarterin CHF millions, except EPS (CHF) and number of shares (millions)2024202320242023Net revenues531312680Operating expenses(305)(517)(106)(124)Operating income (loss)(248)(386)(78)(44)Net income (loss)(258)(420)(75)(51)Basic EPS(1.43)(2.36)(0.41)(0.29)Basic weighted average number of shares180.5178.2182.4178.4Diluted EPS(1.43)(2.36)(0.41)(0.29)Diluted weighted average number of shares180.5178.2182.4178.4 * Idorsia measures, reports, and issues guidance on non-GAAP operating performance. Idorsia believes that these non-GAAP financial measurements more accurately reflect the underlying business performance and therefore provide useful supplementary information to investors. These non-GAAP measures are reported in addition to, not as a substitute for, US GAAP financial performance. Non-GAAP net loss in the first nine months of 2024 amounted to CHF 258 million: the CHF 78 million difference versus US GAAP net loss was mainly due to the one-off effect of the Viatris deal (CHF 125 million income), depreciation and amortization (CHF 14 million), share-based compensation (CHF 17 million) and a consent fee paid in shares to the bondholders resulting from amended terms of the 2024 convertible bonds (CHF 14 million). The non-GAAP net loss resulted in a net loss per share of CHF 1.43 (basic and diluted) in the first nine months of 2024, compared to a net loss per share of CHF 2.36 (basic and diluted) in the first nine months of 2023. Viatris collaborationIn March 2024, Idorsia closed agreements with Viatris Inc. (NASDAQ: VTRS), a global healthcare company, for collaboration on the global development and commercialization of two Phase 3 assets – selatogrel and cenerimod – with Idorsia receiving an upfront payment of USD 350 million, and the right to potential development and regulatory milestone payments of up to USD 300 million, potential sales milestone payments of up to USD 2.1 billion, and potential contingent tiered royalties from mid-single- to low-double-digit percentage on annual net sales. A joint development committee is overseeing the development of the ongoing Phase 3 programs for selatogrel and cenerimod up to regulatory approval. Idorsia will contribute up to USD 200 million in the next 3 years and transferred the dedicated personnel for both programs to Viatris. Viatris has worldwide commercialization rights for both selatogrel and cenerimod (excluding, for cenerimod only, Japan, South Korea, and certain countries in the Asia-Pacific region). Idorsia has also granted Viatris a right of first refusal and first negotiation for certain other pipeline assets. Convertible bonds 2024In July 2018, the Group issued CHF 200 million of senior unsecured convertible bonds (ISIN: CH0426820350), which were due to mature on July 17, 2024. On May 6, 2024, a bondholder meeting was held, where 83.5% of the total outstanding bondholders voted in favor of amendments to the terms of the bonds. The approved bond terms include an amended conversion price of CHF 6.00, extended maturity date of January 17, 2025, and the option to call the bonds at par, in full or in part, at any time upon giving ten trading days' notice. A consent fee of 8,000 shares per Bond was paid to bondholders on September 5, 2024. Financial outlook 2024For 2024 – excluding unforeseen events – the company expects QUVIVIQ net sales of around CHF 55 million; SG&A expenses of around CHF 265 million; R&D expense of around CHF 130 million for Idorsia-led pipeline assets; non-GAAP operating expenses around CHF 400 million. This performance would result in a non-GAAP operating loss of around CHF 350 million (excluding contract revenues and the one-off benefit from the Viatris deal). The company expects US GAAP operating loss for 2024 to reach CHF 260 million which includes a one-off benefit of CHF 125 million from the Viatris deal. Arno Groenewoud, Chief Financial Officer, commented:“While closing a deal for TRYVIO is a key focal point, we have also sharpened our cost-conscious approach, which we will increase going forward. Hence, we have been able to stretch the cash runway out to about year-end 2024, which allows us to appropriately plan and execute the next steps of our financial strategy. Furthermore, as a result of lower than expected spending we can upgrade our US GAAP and non-GAAP operating loss guidance by around 60 million Swiss francs each, taking them to 260 million and 350 million Swiss francs, respectively.” Liquidity and indebtednessAt the end of the first nine months of 2024, Idorsia’s liquidity amounted to CHF 92 million. (in CHF millions)Sep 30, 2024Jun 30, 2024Dec 31, 2023Liquidity Cash and cash equivalents92237145Total liquidity*92237145 Indebtedness Convertible loan335335335Convertible bond797797796Other financial debt162162162Total indebtedness1,2941,2941,293 *rounding differences may occur Commercial operationsIn the first nine months of 2024, QUVIVIQ™ (daridorexant) in the US, Germany, Italy, Switzerland, Spain, UK, Canada, Austria, and France generated total product sales of CHF 39 million. United States ProductMechanism of actionIndicationCommercially available sinceDual orexin receptor antagonistTreatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenanceMay 2022 In the US, net sales of QUVIVIQ® (daridorexant) in the first nine months of 2024 reached CHF 21 million, an increase of +41% versus the first nine months of 2023. As of the end of the third quarter 2024, more than 165,000 patients have been treated with QUVIVIQ since launch in the US, over 500,000 prescriptions have been dispensed, and the product has been prescribed by almost 50,000 healthcare professionals. Tosh Butt, President, and General Manager of Idorsia US, commented:“Due to budget constraints we have recently reduced our field force for QUVIVIQ. Despite the reduction, the sales are holding-up for now. Importantly, the citizen petition requesting a review of the evidence from available data, which will hopefully lead to the descheduling of the DORA class of chronic insomnia medications, continues to make progress.” For more information about QUVIVIQ in the US, see the Full Prescribing Information (PI and Medication Guide). ProductMechanism of actionIndicationCommercially available sinceDual endothelin receptor antagonistTreatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugsOctober 2024 On March 19, 2024, the US Food and Drug Administration (FDA) approved TRYVIO™ (aprocitentan) for the treatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugs. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. The recommended dosage of TRYVIO is 12.5 mg orally once daily, with or without food. Tosh Butt concluded:“Idorsia is making robust progress with the preparation of everything required for a full commercial launch of TRYVIO by the end of the first quarter of 2025. Both the REMS program and specialty distribution channel are fully up and running, we have begun engaging with hypertension experts through our presence at major cardiovascular and nephrology congresses. The initial discussions with payors are also encouraging. TRYVIO is now available to prescribe to the millions of patients in the US whose high blood pressure is not adequately controlled by other drugs. We have everything in place except a field-force and funding for promotional activities, which is dependent on a partnership deal.” Further details on the approval, together with commentary from company management can be found in the dedicated press release and investor webcast available from the company corporate website. For more information see the Full Prescribing Information including BOXED Warning (PI and Medication Guide). Europe and Canada ProductMechanism of actionIndicationCommercially availableDual orexin receptor antagonistTreatment of adult patients with insomnia characterised by symptoms present for at least three months and considerable impact on daytime functioningSweden: Sept. 2024France: Mar. 2024Austria: Feb. 2024UK: Oct. 2023Spain: Sept. 2023Switzerland: Jun. 2023Germany: Nov. 2022Italy: Nov. 2022 Management of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenanceCanada: Nov. 2023 QUVIVIQ (daridorexant) net sales in the first nine months of 2024 reached CHF 18 million in the EUCAN region. In the third quarter of 2024, net sales have increased by 46% compared to the second quarter of 2024. In Germany, QUVIVIQ was launched in November 2022. By law, sleep medications were then subject to a 4-week prescribing limitation (Anlage III BtMG). Following a review by the Federal Joint Committee (G-BA) this limitation was lifted for QUVIVIQ in November 2023. This makes it the only sleep medication in Germany that can be prescribed for long-term treatment of chronic insomnia. In December 2023, the price negotiated for QUVIVIQ under the AMNOG process became effective. Following the lifting of the prescribing limitation, the company submitted a second AMNOG dossier for the long-term treatment of chronic insomnia disorder (beyond 4 weeks), reflecting the indication approved by the EMA in 2022. The second AMNOG process is expected to end in March 2025. The progress made in Germany is reflected by the performance of QUVIVIQ on the market, with sales doubling in the first nine months of 2024 compared to the first nine months of 2023. In Italy, QUVIVIQ was launched in November 2022. Currently, QUVIVIQ can only be prescribed by neurologists, psychiatrists, and specialists from sleep centers, and no sleep therapy is reimbursed. The company submitted a reimbursement dossier in June 2023 and requested the expansion of the prescriber base. The submission – detailing the efficacy and safety profile of QUVIVIQ and its estimated budget impact and cost-effectiveness in Italy – is under review, with a hearing expected to take place before the end of the year. In Switzerland, QUVIVIQ was launched to the self-pay market in June 2023. Following the launch of QUVIVIQ, awareness and sales have increased solidly. Reimbursement discussions are ongoing and remain a priority for Switzerland. In Spain, QUVIVIQ was launched to the self-pay market in September 2023. Spain represents the largest insomnia market in Europe, as was apparent in the first months of this product’s availability, despite it only being launched to the self-pay market. The company submitted a reimbursement dossier to the Spanish authorities in July 2024, in order to allow equal access for all patients with chronic insomnia. In the UK, QUVIVIQ is recommended as first-line pharmaceutical treatment for patients with chronic insomnia, after, or as an alternative to, cognitive behavioral therapy for insomnia (CBT-I). QUVIVIQ was launched in October 2023 and the team has achieved full reimbursement throughout the UK. The priority in the UK is to secure regional access, which currently stands at around 80%, as well as raising awareness of QUVIVIQ among general practitioners. In Austria, QUVIVIQ was made available in February 2024. A reimbursement dossier was submitted in October 2024, with a conclusion expected in the second half of 2025. In France, QUVIVIQ was included in both the hospital and the retail formulary list of reimbursed pharmaceutical specialties in January 2024 and launched in March 2024 as the first and only pharmacotherapy recommended for the treatment of chronic insomnia disorder. There was a very strong uptake at launch largely due to the excellent market preparation work with psychiatrists. This can be seen by the fact that the majority of prescriptions are being made by the specialists, despite the French market being composed of 55’000 general practitioners who between them represent 75% of the insomnia prescriptions. As a result, the priority becomes expanding awareness to the primary care market to secure strong long-term growth. To address this, Idorsia is expanding its commercial reach from specialist prescribers to general practitioners (GPs) through a new commercial partnership with Menarini in France. In Sweden, QUVIVIQ was made available in September 2024. A reimbursement dossier was submitted in May 2024. As a result, a decision on reimbursement is expected by the end of 2024. In Canada, after being approved in April 2023, QUVIVIQ was launched in November 2023 to the private market, representing 55% of the Canadian insomnia market. The reimbursement dossier was submitted to private market payers in the third quarter of 2023 and currently stands at 80% coverage. The focus is now on public payers with the submission confirmation in Quebec received in October 2024 and submissions in all other provinces ongoing and to be completed by year end. Benjamin Limal, President of Europe and Canada region, commented:“Securing public access to Europe’s only dual orexin receptor antagonist remains our number one priority throughout the EUCAN region – this is the pathway to unlocking the true value of QUVIVIQ. Until then, the performance is satisfactory with a great adoption from specialists and a strong quarterly growth in demand and sales. As one of the newest launches, I have to mention France – QUVIVIQ is really off to a flying start and we are quickly reinforcing the launch momentum through a commercial partnership with Menarini, experts with established relationships in primary care, to handle the promotion of QUVIVIQ to GPs.” For more information about QUVIVIQ in the EU, see the Summary of Product Characteristics. For more information about QUVIVIQ in Switzerland, see the Patient Information and Information for Healthcare Professionals. For more information on the marketing authorization of QUVIVIQ in Canada, see the Product Monograph. ProductMechanism of actionIndicationCommercially available sinceDual endothelin receptor antagonistTreatment of resistant hypertension in adult patients in combination with at least three antihypertensive medicinal productsApproved Jun. 2024 On June 27, 2024, the European Commission (EC) approved JERAYGO™ (aprocitentan) for the treatment of resistant hypertension in adult patients in combination with at least three antihypertensive medicinal products. The recommended dose is 12.5 mg orally once daily. The dose can be increased to 25 mg once daily for patients tolerating the 12.5 mg dose and in need of tighter blood pressure (BP) control. Further details on the approval, together with commentary from company management can be found in the dedicated press release available from the company corporate website. For more information about JERAYGO in the EU, see the Summary of Product Characteristics. Research & DevelopmentIdorsia has a diversified and balanced portfolio, comprising assets developed and/or marketed by Idorsia and assets that are partner-led to maximize the value we have created. Our drug discovery engine has produced innovative drugs with the potential to transform the treatment paradigm in multiple therapeutic areas, including CNS, cardiovascular, and immunological disorders, as well as orphan diseases. The company also has a vaccine platform for the discovery and development of glycoconjugate vaccines containing synthetic antigenic glycan molecules, with or without a carrier protein, to prevent infection. Idorsia-led portfolio CompoundMechanism of actionTarget indicationStatusQUVIVIQ™ (daridorexant)Dual orexin receptor antagonistInsomniaCommercially available in the US, Germany, Italy, Switzerland, Spain, the UK, Canada, Austria, France, and Sweden; approved throughout the EUTRYVIO™ (aprocitentan) Dual endothelin receptor antagonistSystemic hypertension in combination with other antihypertensivesCommercially available in the USJERAYGO™ (aprocitentan)Dual endothelin receptor antagonistResistant hypertension in combination with other antihypertensivesApproved in the EU; Marketing authorization applications submitted in the UK, Canada, and SwitzerlandLucerastatGlucosylceramide synthase inhibitorFabry diseasePhase 3 primary endpoint not met; open-label extension study ongoingPhase 3 focused on renal function in preparationDaridorexantDual orexin receptor antagonistPediatric insomniaPhase 2 in pediatric insomnia ongoingACT-1004-1239ACKR3/CXCR7 antagonistDemyelinating diseases including multiple sclerosis Phase 2 in preparationACT-777991CXCR3 antagonistVitiligoPhase 2 in preparationSinbaglustatGBA2/GCS inhibitorRare lysosomal storage disordersPhase 1 completeIDOR-1117-2520UndisclosedImmune-mediated disordersPhase 1 ongoingIDOR-1134-2831Synthetic glycan vaccineClostridium difficile infectionPhase 1 ongoing Further details including the current status of each project in our portfolio can be found in our innovation fact sheet. Idorsia partner-led portfolioFor Idorsia, sophisticated partnerships are a way of gaining strategic access to technologies or products and fully exploiting our discovery engine and clinical pipeline. We seek suitable external project partners to maximize the value of internal innovation. CompoundMechanism of actionTarget indicationPartner/statusQUVIVIQ™ (daridorexant)Dual orexin receptor antagonistInsomniaSimcere: Approved for the treatment of insomnia in Hong-KongQUVIVIQ™ (daridorexant)Dual orexin receptor antagonistInsomniaNxera Pharma: license to develop and commercialize for Asia-Pacific region (excluding China)Approved for the treatment of insomnia in JapanDaridorexantDual orexin receptor antagonistInsomniaSimcere: license to develop and commercialize for Greater China regionNDA submitted in Greater ChinaSelatogrelP2Y12 inhibitorAcute myocardial infarctionViatris: worldwide development and commercialization rightsPhase 3 “SOS-AMI” program ongoingCenerimodS1P1 receptor modulatorSystemic lupus erythematosusViatris: worldwide development and commercialization rights (excluding Japan, South Korea, and certain countries in the Asia-Pacific region)Phase 3 “OPUS” program ongoingDaridorexantDual orexin receptor antagonistPosttraumatic stress disorder (PTSD)US Department of Defense (DOD): Idorsia is supporting a clinical study sponsored by the US DOD to develop new therapies to treat PTSDACT-1002-4391EP2/EP4 receptor antagonistImmuno-oncologyOwkin: global license to develop and commercializePhase 1 in preparation On October 1, 2024, Nxera Pharma announced that it had entered a commercial partnership agreement with Shionogi & Co., Ltd regarding the distribution and sales for QUVIVIQ in Japan. At the same time, the previous commercialization agreement between Nxera and Mochida Pharmaceutical Co., Ltd. was terminated. After negotiation among Nxera, Shionogi and Mochida regarding the optimal sales scheme, Shionogi is solely responsible for distribution and sales activities in Japan. Idorsia and Neurocrine Biosciences had a collaboration for ACT-709478, Idorsia’s novel T-type calcium channel blocker. The compound was investigated as a treatment of pediatric patients with epileptic encephalopathy with continuous spike-and-wave during sleep (CSCW), a rare form of pediatric epilepsy. The Phase 2 study did not meet the primary endpoint in June 2022, and further analysis from an open-label extension study resulted in the decision to stop further development. As a result, the development agreement has come to an end. Further details including the current status of each project in our partner-led portfolio can be found in our innovation fact sheet. Results Day CenterInvestor community: To make your job easier, we provide all relevant documentation via the Results Day Center on our corporate website: www.idorsia.com/results-day-center. Upcoming Financial Updates Full Year 2024 Financial Reporting together with the publication of the Annual Report 2024 on February 27, 2025 Notes to the editor About IdorsiaIdorsia Ltd is reaching out for more – We have more ideas, we see more opportunities and we want to help more patients. In order to achieve this, we will develop Idorsia into a leading biopharmaceutical company, with a strong scientific core. Headquartered near Basel, Switzerland – a European biotech-hub – Idorsia is specialized in the discovery, development, and commercialization of small molecules to transform the horizon of therapeutic options. Idorsia has a 25-year heritage of drug discovery, a broad portfolio of innovative drugs in the pipeline, an experienced team of professionals covering all disciplines from bench to bedside, and commercial operations in Europe and North America – the ideal constellation for bringing innovative medicines to patients. Idorsia was listed on the SIX Swiss Exchange (ticker symbol: IDIA) in June 2017 and has over 750 highly qualified specialists dedicated to realizing our ambitious targets. For further information, please contactAndrew C. WeissSenior Vice President, Head of Investor Relations & Corporate CommunicationsIdorsia Pharmaceuticals Ltd, Hegenheimermattweg 91, CH-4123 Allschwil+41 58 844 10 10investor.relations@idorsia.com media.relations@idorsia.com www.idorsia.com The above information contains certain "forward-looking statements", relating to the company's business, which can be identified by the use of forward-looking terminology such as "estimates", "believes", "expects", "may", "are expected to", "will", "will continue", "should", "would be", "seeks", "pending" or "anticipates" or similar expressions, or by discussions of strategy, plans or intentions. Such statements include descriptions of the company's investment and research and development programs and anticipated expenditures in connection therewith, descriptions of new products expected to be introduced by the company and anticipated customer demand for such products and products in the company's existing portfolio. Such statements reflect the current views of the company with respect to future events and are subject to certain risks, uncertainties and assumptions. Many factors could cause the actual results, performance or achievements of the company to be materially different from any future results, performances or achievements that may be expressed or implied by such forward-looking statements. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described herein as anticipated, believed, estimated or expected.
Attachment
Press Release PDF
Drug ApprovalFinancial Statement
21 May 2024
Ad hoc announcement pursuant to Art. 53 LR Allschwil, Switzerland – May 21, 2024 Idorsia Ltd (SIX: IDIA) today announced its financial results for the first quarter of 2024. Business highlights Viatris collaboration: Global research and development collaboration, focused on the development and commercialization of two innovative compounds, selatogrel and cenerimod. Commercial highlights QUVIVIQ™ (daridorexant): Total net sales of CHF 10 million in Q1 2024.QUVIVIQ in the US: Citizens petition to deschedule the DORA class progressing.QUVIVIQ in Europe: Further launches, including France, provide a solid base to increase European sales in 2024. Pipeline highlights TRYVIO™ (aprocitentan): Approved by the US FDA in March 2024.JERAYGO™ (aprocitentan): Recommended for approval in Europe.Daridorexant: Phase 3 study conducted by Simcere in Chinese patients fully recruited. Financial highlights Net revenue Q1 2024 at CHF 10 million.US GAAP operating expenses Q1 2024 benefiting from extraordinary income from Viatris deal with non-GAAP operating expenses Q1 2024 at CHF 96 million.US GAAP operating income Q1 2024 of CHF 31 million and non-GAAP operating loss of CHF 85 million.The Viatris deal: The upfront consideration of USD 350 million (CHF 308 million) was fully paid by Viatris to Idorsia in Q1 2024.Convertible bond 2024: Bondholders approve an extension of maturity by six months. Guidance for 2024 QUVIVIQ net sales of around CHF 55 million.US GAAP operating loss to reach CHF 340 million (which includes a one-off benefit of CHF 125 million from the Viatris deal), non-GAAP operating loss of around CHF 420 million (excluding contract revenues and the one-off benefit from the Viatris deal) – unforeseen events excluded. Jean-Paul Clozel, MD and Chief Executive Officer, commented:“We have already reached significant milestones in 2024. The deal with Viatris to accelerate the development of selatogrel and cenerimod brought cash and security for these assets, while retaining shareholder value. We came to an arrangement with holders of the convertible bond 2024 due for repayment in July, giving us the time we need to secure additional funding and avoid liquidity constraints. Also, the quality of our research and development engine was once again confirmed by the FDA’s approval of TRYVIO (aprocitentan) and the CHMP positive opinion received for JERAYGO (the trade name of aprocitentan in Europe). This will now unlock value for Idorsia as we evaluate possible launch strategies – including potential partnership – for the first antihypertensive working on a new pathway seen in almost 40 years.” Jean-Paul continued: “Since the first launch of QUVIVIQ, more than 14 million tablets have been dispensed worldwide, with well over 150,000 patients benefiting from QUVIVIQ. We continue to believe in the huge potential offered by this product and – thanks to a long patent life – there is plenty of time for this potential to be realized. Continued innovation is essential to securing the company’s future. Despite the reduction of the workforce, we continue to discover and develop new drugs with great potential in many areas of medicine. I am very confident that our vision to create an innovative, profitable, and sustainable science-based company will become a reality in the coming years.” Financial results US GAAP results First Quarterin CHF millions, except EPS (CHF) and number of shares (millions) 20242023Net revenues 1021Operating expenses 20(219)Operating income (loss) 31(198)Net income (loss) 30(212)Basic EPS 0.17(1.19)Basic weighted average number of shares 179.1178.0Diluted EPS 0.13(1.19)Diluted weighted average number of shares 233.3178.0 Net revenue of CHF 10 million in the first quarter of 2024 is the result of QUVIVIQ product sales. This compares to CHF 21 million in the first quarter of 2023, which included CHF 13.5 million sales of PIVLAZ in Japan (now assigned to Nxera Pharma as part of a transaction, more details can be found in the dedicated press release) and CHF 1 million revenue share from Johnson & Johnson related to ponesimod sales (revenue-sharing agreement now eliminated as part of the reacquisition of aprocitentan, more details can be found in the dedicated press release). US GAAP operating expenses in the first quarter of 2024 benefitted from extraordinary income of CHF 125 million from the Viatris deal resulting in a negative expense of CHF 20 million (CHF 219 million in the first quarter of 2023), of which CHF 4 million related to cost of sales (CHF 1 million in the first quarter of 2023), CHF 33 million to R&D expenses (CHF 93 million in the first quarter of 2023), and CHF 68 million to SG&A expenses (CHF 125 million in the first quarter of 2023). US GAAP net income in the first quarter of 2024 amounted to CHF 30 million (CHF 212 million net loss in the first quarter of 2023). The decrease of the net loss is mainly attributable to the one-off income related to the Viatris Deal but was also driven by lower operating expenses throughout all functions. The US GAAP net income resulted in a basic net income per share of CHF 0.17 (diluted net income per share of CHF 0.13) in the first quarter of 2024, compared to a net loss per share of CHF 1.19 (basic and diluted) in the first quarter of 2023. Non-GAAP* measures First Quarterin CHF millions, except EPS (CHF) and number of shares (millions) 20242023Net revenues 1021Operating expenses (96)(202)Operating income (loss) (85)(181)Net income (loss) (86)(189)Basic EPS (0.48)(1.06)Basic weighted average number of shares 179.1178.0Diluted EPS (0.48)(1.06)Diluted weighted average number of shares 179.1178.0 * Idorsia measures, reports, and issues guidance on non-GAAP operating performance. Idorsia believes that these non-GAAP financial measurements more accurately reflect the underlying business performance and therefore provide useful supplementary information to investors. These non-GAAP measures are reported in addition to, not as a substitute for, US GAAP financial performance. Non-GAAP net loss in the first quarter of 2024 amounted to CHF 86 million: the CHF 116 million difference versus US GAAP net income was mainly due to the one-off effect of the Viatris Deal (CHF 125 million income), depreciation and amortization (CHF 4 million), and share-based compensation (CHF 4 million). The non-GAAP net loss resulted in a net loss per share of CHF 0.48 (basic and diluted) in the first quarter of 2024, compared to a net loss per share of CHF 1.06 (basic and diluted) in the first quarter of 2023. Viatris collaborationIn March 2024, Idorsia closed agreements with Viatris Inc. (NASDAQ: VTRS), a global healthcare company, for collaboration on the global development and commercialization of two Phase 3 assets – selatogrel and cenerimod – with Idorsia receiving an upfront payment of USD 350 million, and the right to potential development and regulatory milestone payments of up to USD 300 million, potential sales milestone payments of up to USD 2.1 billion, and potential contingent tiered royalties from mid-single- to low-double-digit percentage on annual net sales. A joint development committee is overseeing the development of the ongoing Phase 3 programs for selatogrel and cenerimod up to regulatory approval. Idorsia will contribute up to USD 200 million in the next 3 years and transferred the dedicated personnel for both programs to Viatris. Viatris has worldwide commercialization rights for both selatogrel and cenerimod (excluding, for cenerimod only, Japan, South Korea, and certain countries in the Asia-Pacific region). Idorsia has also granted Viatris a right of first refusal and first negotiation for certain other pipeline assets. Convertible bonds 2024In July 2018, the Group issued CHF 200 million of senior unsecured convertible bonds (ISIN: CH0426820350), which were due to mature on July 17, 2024. On May 6, 2024, a bondholder meeting was held, where 83.5% of the total outstanding bondholders voted in favor of amendments to the terms of the bonds. The approved bond terms include an amended conversion price of CHF 6.00, extended maturity date of January 17, 2025, and the option to call the bonds at par, in full or in part, at any time upon giving ten trading days' notice. The company has applied to the higher cantonal composition authority and upon approval the amendments to the bond terms will become binding and effective. A consent fee of 8,000,000 Idorsia shares will be delivered through SIX SIS once the amendment of the bond terms is effective. Financial outlook 2024For 2024 – excluding unforeseen events – the company expects QUVIVIQ net sales of around CHF 55 million; SG&A expenses of around CHF 300 million; R&D expense of around CHF 165 million for Idorsia-led pipeline assets; non-GAAP operating expenses of up to CHF 470 million. This performance would result in a non-GAAP operating loss of around CHF 420 million (excluding contract revenues and the one-off benefit from the Viatris deal). The company expects US GAAP operating loss for 2024 to reach CHF 340 million which includes a one-off benefit of CHF 125 million from the Viatris deal. André C. Muller, Chief Financial Officer, commented:“In addition to the funds already raised from our business development activities, I am confident in our ability to raise additional funding this year. We will continue to evaluate and prepare possible launch strategies – including potential partnership – for TRYVIO. The significant progress with access and availability of QUVIVIQ has started to gain traction, particularly in Europe, this will translate into higher sales in 2024. At the same time, the cost reduction initiative that took place in the latter part of 2023 is fully effective and reflected in our 2024 guidance, with significantly lower expenses. We must continue to control our costs and explore all avenues to extend our cash runway, but I see many reasons to be optimistic for the future of Idorsia.” Liquidity and indebtednessAt the end of the first quarter of 2024, Idorsia’s liquidity amounted to CHF 335 million. (in CHF millions) March 31, 2024Dec 31, 2023Liquidity Cash and cash equivalents 335145Short-term deposits --Total liquidity* 335145 Indebtedness Convertible loan 335335Convertible bond 797796Other financial debt 162162Total indebtedness 1,2931,293 *rounding differences may occur Commercial operationsIn the first quarter of 2024, QUVIVIQ™ (daridorexant) in the US, Germany, Italy, Switzerland, Spain, UK, Canada, Austria, and France generated total product sales of CHF 10 million. United States ProductMechanism of actionIndicationCommercially available sinceDual orexin receptor antagonistTreatment of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenanceMay 2022 In the US, net sales of QUVIVIQ® (daridorexant) in the first quarter of 2024 reached CHF 6.5 million. This net sales number includes the QUVIVIQ copay program aimed at driving demand and product uptake, and thus does not reflect the actual number of prescriptions dispensed. As of the end of the first quarter of 2024, more than 140,000 patients have been treated with QUVIVIQ, almost 400,000 prescriptions have been dispensed, and the product has been prescribed by more than 42,000 healthcare professionals. To begin with, the company ran a direct-to-consumer (DTC) television and digital campaign and offered a copay program. The strategy was to create a recognizable brand, enabling market access discussions. During 2023, the company made significant progress, reaching over 65% reimbursement in the commercial sector. As access increased, the commercial approach was adjusted, with the aim being to switch from a consignment model (providing substantially reduced or free prescriptions) to a payer paid model. In the first quarter of 2024, paid prescriptions accounted for 68% of the total – an increase of 36 percentage points from the same period in 2023 and of 7 percentage points from the previous quarter. The first Medicare Part D coverage – reaching 27% of covered lives – began in January 2024, opening an entirely new channel which has the potential to substantially improve product access and paid prescriptions. In February, there was a cyberattack on Change Healthcare (UnitedHealth Group), the largest adjudicator/processor of copay cards in the US, causing major disruption across the pharmaceutical industry, including the QUVIVIQ copay cards, with a negative impact on prescription dispensing levels. In March, the Idorsia US Market Access team put a solution in place to remedy the disruption, though the impact was still appreciable through to the end of March. In April 2023, Idorsia filed a citizen petition (CP), urging the Drug Enforcement Administration (DEA) to deschedule the DORA class of chronic insomnia medications, based on a review of evidence from available data, including post-marketing surveillance data. Starting in 2015, the independent FDA approvals of other DORAs included a recommendation that these drug products be scheduled based on preclinical data. The CP to deschedule the DORA class outlines current scientific and medical evidence demonstrating that the DORA class has a negligible abuse profile and potential for abuse, lacks non-medical use in the community, lacks physical and psychological dependence, and therefore, should not be a scheduled class under the Controlled Substances Act. The DEA and FDA acknowledged the CP, and the process to analyze and examine the request is moving forward. Notably, a report accompanying the FDA appropriations bill that was finalized in March 2024 informed the FDA that the process for descheduling the DORA class is a priority for Congress. Tausif ‘Tosh’ Butt, President, and General Manager of Idorsia US, commented:“I believe one of the biggest barriers to prescribing QUVIVIQ is the fact it is currently a scheduled drug. Apart from the obstacles to prescribing scheduled drugs, some payers require patients to be treated with low-cost drugs not indicated for insomnia, and others that carry black box warnings before covering QUVIVIQ. The US Congress has long supported the efforts of the FDA to address the opioid and addiction crisis, and this year it encouraged the FDA to also consider the impact of treatments for insomnia as a part of that larger public health mission. I am very hopeful for our citizen petition requesting a review of the evidence can lead to the descheduling of the DORA class of chronic insomnia medications.” For more information about QUVIVIQ in the US, see the Full Prescribing Information (PI and Medication Guide). ProductMechanism of actionIndicationCommercially available sinceDual endothelin receptor antagonistTreatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugsApproved Mar. 2024Planned availability: H2 2024 On March 19, 2024, the US Food and Drug Administration (FDA) approved TRYVIO™ (aprocitentan) for the treatment of hypertension in combination with other antihypertensive drugs, to lower blood pressure in adult patients who are not adequately controlled on other drugs. Lowering blood pressure reduces the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. The recommended dosage of TRYVIO is 12.5 mg orally once daily, with or without food. Idorsia plans to make TRYVIO available in the second half of 2024 to the millions of patients in the US whose high blood pressure is not adequately controlled by other drugs. Further details on the approval, together with commentary from company management can be found in the dedicated press release and investor webcast available from the company corporate website. For more information see the Full Prescribing Information including BOXED Warning (PI and Medication Guide). Europe and Canada ProductMechanism of actionIndicationCommercially availableDual orexin receptor antagonistTreatment of adult patients with insomnia characterised by symptoms present for at least three months and considerable impact on daytime functioningFrance: Mar. 2024Austria: Feb. 2024UK: Oct. 2023Spain: Sep. 2023Switzerland: Jun. 2023Germany: Nov. 2022Italy: Nov. 2022 Management of adult patients with insomnia, characterized by difficulties with sleep onset and/or sleep maintenanceCanada: Nov. 2023 QUVIVIQ (daridorexant) net sales in the first quarter of 2024 reached CHF 3.5 million in the EUCAN region. In November 2023, treatment with daridorexant was added to the insomnia treatment guidelines for Europe. In “The European Insomnia Guideline: An update on the diagnosis and treatment of insomnia 2023”, published in the Journal of Sleep Research, the authors note that “The introduction of DORAs has probably been the most significant recent development in the pharmacological treatment of insomnia.” In Germany, QUVIVIQ was launched in November 2022. By law, sleep medications were then subject to a 4-week prescribing limitation (Anlage III BtMG). Following a review by the Federal Joint Committee (G-BA) – the highest decision-making body of the joint self-government of physicians, dentists, hospitals, and health insurance funds in Germany – this limitation was lifted for QUVIVIQ in November 2023. This makes it the only sleep medication in Germany that can be prescribed for long-term treatment of chronic insomnia. In December 2023, the price negotiated for QUVIVIQ under the AMNOG process became effective. Following the lifting of the prescribing limitation, the company submitted a second AMNOG dossier for the long-term treatment of chronic insomnia disorder (beyond 4 weeks), reflecting the indication approved by the EMA in 2022. The progress made in Germany is reflected by the performance of QUVIVIQ on the market, with a 63% increase in demand seen in Q4 2023 (compared to Q3 2023), followed by a strong start to 2024 (February +41% compared to December 2023). In Italy, QUVIVIQ was launched in November 2022. Currently, QUVIVIQ can only be prescribed by neurologists, psychiatrists, and specialists from sleep centers, and no sleep therapy is reimbursed. The company submitted a reimbursement dossier in June 2023 and requested the expansion of the prescriber base. The submission – detailing the efficacy and safety profile of QUVIVIQ and its estimated budget impact and cost-effectiveness in Italy – is under review, with the final outcome expected in the second half of 2024. In Switzerland, QUVIVIQ was launched to the self-pay market in June 2023. Following the launch of QUVIVIQ, awareness has increased among all specialties, and demand has increased solidly (+32% in Q4 2023 compared to Q3 2023) ahead of reimbursement, which is expected in the summer of 2024. In Spain, QUVIVIQ was launched to the self-pay market in September 2023. Spain represents the largest insomnia market in Europe, as was apparent in the first months of this product’s availability, despite it only being launched to the self-pay market. The company is assessing the opportunity to submit a reimbursement dossier to the Spanish authorities, in order to allow equal access for all patients with chronic insomnia. In the UK, QUVIVIQ was launched in October 2023. At the same time, technology appraisal guidance was published by the National Institute for Health and Care Excellence (NICE), allowing the transition to local access discussions and listing by healthcare boards for England, Wales, and Northern Ireland. In April 2024, the Scottish Medicines Consortium (SMC) also accepted QUVIVIQ for use within NHS Scotland. This means that the company has achieved full reimbursement throughout the UK, where QUVIVIQ is now recommended as first-line pharmaceutical treatment for patients with chronic insomnia, after, or as an alternative to, cognitive behavioral therapy for insomnia (CBT-I). The priority in the UK now, is to secure regional access. In France, QUVIVIQ was launched in March 2024 as the first and only pharmacotherapy recommended for the treatment of chronic insomnia disorder. In January 2024, the inclusion of QUVIVIQ in both the hospital and the retail formulary list of reimbursed pharmaceutical specialties was announced in the French Official Gazette, together with the French public price. This official publication means that, with a prescription from their doctor, patients with chronic insomnia in France have access to the treatment if they meet the requirements of the EU prescribing label for QUVIVIQ. The publication follows the positive recommendation by the Transparency Committee in May 2023, recognizing QUVIVIQ as providing clinical added value. In Canada, after being approved in April 2023, QUVIVIQ was launched in November 2023 to the private market, representing 55% of the Canadian insomnia market. The reimbursement dossier was submitted to private market payers in the third quarter of 2023, and just a few months after the submission the team had secured reimbursement for more than 60% of private market patients. The focus is now on public payers with the submission to INESSS (Institut national d’excellence en santé et en services sociaux) finalized in March 2024 and the submission to CADTH (Canada’s Drug and Health Technology Agency) expected in the second quarter of 2024. Jean-Yves Chatelan, President of Europe and Canada region, commented:“The launch of Europe’s first and only dual orexin receptor antagonist is progressing well across all markets where we have made QUVIVIQ available. Including Canada, we have expanded availability into more markets and improved the reimbursement environment beyond many expectations. With continued positive feedback from physicians and patients on the differentiated profile of QUVIVIQ, I am very optimistic that the progress we have made will now translate into many more patients benefiting from QUVIVIQ and increasing volumes advancing the region towards profitability.” For more information about QUVIVIQ in the EU, see the Summary of Product Characteristics. For more information about QUVIVIQ in Switzerland, see the Patient Information and Information for Healthcare Professionals. For more information on the marketing authorization of QUVIVIQ in Canada, see the Product Monograph. Research & DevelopmentIdorsia has a diversified and balanced portfolio, comprising assets developed and/or marketed by Idorsia and assets that are partner-led to maximize the value we have created. Our drug discovery engine has produced innovative drugs with the potential to transform the treatment paradigm in multiple therapeutic areas, including CNS, cardiovascular, and immunological disorders, as well as orphan diseases. The company also has a vaccine platform for the discovery and development of glycoconjugate vaccines containing synthetic antigenic glycan molecules, with or without a carrier protein, to prevent infection. Alberto Gimona, MD and Head of Global Clinical Development of Idorsia, commented:“Despite a difficult period for our organization, the team has shown extraordinary commitment and made great progress with our portfolio. This is particularly evident in the successful registration of aprocitentan in the US and the positive opinion from the European Union’s CHMP, with labels that reflect the value of the compound. I was also very pleased to have found a way for both selatogrel and cenerimod programs to be fully supported through the collaboration with Viatris, while maintaining our involvement in their development. I look forward to advancing the portfolio and bringing benefits to patients in many areas of medical need.” Idorsia-led portfolio CompoundMechanism of actionTarget indicationStatusQUVIVIQ™ (daridorexant)Dual orexin receptor antagonistInsomniaCommercially available as QUVIVIQ in the US, Germany, Italy, Switzerland, Spain, the UK, Canada, Austria, and France; approved throughout the EUTRYVIO™ (aprocitentan) Dual endothelin receptor antagonistSystemic hypertension in combination with other antihypertensivesApproved as TRYVIO in the US, launch planned for H2 2024JERAYGO™ (aprocitentan)Dual endothelin receptor antagonistResistant hypertension in combination with other antihypertensivesPositive opinion from the European Committee for Medicinal Products for Human Use (CHMP) received in April 2024 – European Commission decision expected in approx. 2 monthsLucerastatGlucosylceramide synthase inhibitorFabry diseasePhase 3 primary endpoint not met; open-label extension study ongoingPhase 3 focused on renal function in preparationDaridorexantDual orexin receptor antagonistPediatric insomniaPhase 2 in pediatric insomnia ongoingACT-1004-1239ACKR3/CXCR7 antagonistDemyelinating diseases including multiple sclerosis Phase 2 in preparationSinbaglustatGBA2/GCS inhibitorRare lysosomal storage disordersPhase 1 completeACT-777991CXCR3 antagonistRecent-onset Type 1 diabetesPhase 1 completeIDOR-1117-2520UndisclosedImmune-mediated disordersPhase 1 ongoingIDOR-1134-2831Synthetic glycan vaccineClostridium difficile infectionPhase 1 initiating DaridorexantDaridorexant is a dual orexin receptor antagonist (DORA) which blocks the binding of the wake-promoting orexin neuropeptides. Rather than inducing sleep through broad inhibition of brain activity, daridorexant only blocks the activation of orexin receptors. Daridorexant is commercially available as QUVIVIQ in the US, Germany, Italy, Switzerland, Spain, the UK, Canada, Austria, and France, and is approved throughout the EU (see “Commercial operations” above). A post-approval study to investigate the efficacy of daridorexant in patients with insomnia and comorbid nocturia has completed recruitment and is expected to report results in mid-2024 (NCT05597020). Idorsia has initiated a Phase 2 dose-finding study to assess the efficacy, safety, and pharmacokinetics of multiple-dose oral administration of daridorexant in pediatric patients aged 10 to <18 years with insomnia disorder (NCT05423717). The primary objective of the study is to characterize the dose-response relationship of daridorexant on objective total sleep time (TST), using polysomnography. The study is expected to enroll around 150 patients, who will be randomized in a 1:1:1:1 ratio to 10 mg, 25 mg, or 50 mg daridorexant, or placebo. The study is part of a US FDA-approved Pediatric Study Plan and an EU PDCO-approved Paediatric Investigation Plan. Aprocitentan Aprocitentan is a once-daily, orally active, dual endothelin receptor antagonist, which inhibits the binding of ET-1 to ETA and ETB receptors. Aprocitentan has a low potential for drug-drug interaction and a mechanism of action suited for lowering blood pressure in adult patients whose hypertension is not adequately controlled by other drugs. On March 19, 2024, aprocitentan was approved as TRYVIO in the US, with availability planned for H2 2024. On April 25, 2024, Idorsia received a positive opinion for aprocitentan (as JERAYGO™) from the Committee for Medicinal Products for Human Use (CHMP) as a treatment of resistant hypertension. A CHMP positive opinion is one of the final steps before marketing authorization can be granted by the European Commission; a final decision is expected approximately two months after publication of the CHMP opinion. Lucerastat Lucerastat is an oral inhibitor of glucosylceramide synthase, offering a potential new treatment approach for all patients living with Fabry disease, irrespective of the mutation type of the GLA gene. In October 2021, the company reported that lucerastat 1000 mg b.i.d. did not meet the primary endpoint of reducing neuropathic pain during 6 months of treatment versus placebo. However, Lucerastat demonstrated a substantial reduction in levels of the Fabry disease biomarker plasma Gb3 during the treatment period, with a decrease of approximately 50% observed in plasma Gb3 in the lucerastat treatment group compared to an increase of 12% in the placebo group. Furthermore, results suggested a treatment effect on kidney function. Lucerastat was well tolerated. Analysis of the ongoing open-label extension (OLE) of the Phase 3 study corroborated the long-term effect on plasma Gb3 levels and a potential positive long-term effect on kidney function. The analysis also showed a safety and tolerability profile consistent with that observed during the 6-month randomized treatment period. The company is conducting a kidney biopsy substudy within a subset of patients currently participating in the OLE study in order to steer further development in Fabry disease. Further details including the current status of each project in our portfolio can be found in our innovation fact sheet. Idorsia partner-led portfolioFor Idorsia, sophisticated partnerships are a way of gaining strategic access to technologies or products and fully exploiting our discovery engine and clinical pipeline. We seek suitable external project partners to maximize the value of internal innovation. CompoundMechanism of actionTarget indicationPartner/statusDaridorexantDual orexin receptor antagonistInsomniaNxera Pharma: license to develop and commercialize for Asia-Pacific region (excluding China)NDA submitted in JapanDaridorexantDual orexin receptor antagonistInsomniaSimcere: license to develop and commercialize for Greater China regionPhase 3 ongoingSelatogrelP2Y12 inhibitorAcute myocardial infarctionViatris: worldwide development and commercialization rightsPhase 3 “SOS-AMI” program ongoingCenerimodS1P1 receptor modulatorSystemic lupus erythematosusViatris: worldwide development and commercialization rights (excluding Japan, South Korea, and certain countries in the Asia-Pacific region)Phase 3 “OPUS” program ongoingDaridorexantDual orexin receptor antagonistPosttraumatic stress disorder (PTSD)US Department of Defense (DOD): Idorsia is supporting a clinical study sponsored by the US DOD to develop new therapies to treat PTSDACT-709478 (NBI-827104)T-type calcium channel blockerEpileptic encephalopathy with continuous spike-and-wave during sleep (CSCW)Neurocrine Biosciences: global license to develop and commercializePhase 2 OLE study ongoingACT-1002-4391EP2/EP4 receptor antagonistImmuno-oncologyOwkin: global license to develop and commercializePhase 1 in preparation Daridorexant (Nxera Pharma)Daridorexant is licensed to Nxera Pharma (previously known as Sosei Heptares) in the Asia-Pacific region (excluding China), and a New Drug Application (NDA) is under review with the Japanese Ministry of Health, Labor, and Welfare (MHLW). In Japan, Idorsia has a license agreement with Mochida Pharmaceutical for the supply, co-development and co-marketing of daridorexant. All potential milestones have been assigned to Nxera. Asia-Pacific region (excluding China): Australia, Brunei, Cambodia, Indonesia, Japan, Laos, Malaysia, Myanmar, New Zealand, Philippines, Singapore, South Korea, Thailand, Taiwan, and Vietnam. Daridorexant (Simcere)Daridorexant is licensed to Simcere in the Greater China region (Mainland China, Hong Kong, and Macau), and a Phase 3 study with daridorexant in Chinese patients has completed recruitment. Results are expected in June 2024 and, if the study is successful, an NDA in Mainland China is planned for the second half of 2024. An NDA is already under review with the Hong Kong Department of Health. Selatogrel and cenerimod (Viatris)A joint development committee from Idorsia and Viatris is overseeing the development of two ongoing Phase 3 programs up to regulatory approval. Selatogrel is a potent, fast-acting, reversible, and highly selective P2Y12 inhibitor being developed in a Phase 3 study (NCT04957719) for the treatment of acute myocardial infarction (“SOS-AMI”) in patients with a recent history of AMI. It is intended to be self-administered subcutaneously via a drug delivery system (autoinjector). Cenerimod is a highly selective S1P1 receptor modulator, given as an oral once-daily tablet, which is being developed in a Phase 3 program known as “OPUS” (NCT05648500, NCT05672576) for the treatment of systemic lupus erythematosus (SLE). Viatris has worldwide commercialization rights for both selatogrel and cenerimod (excluding, for cenerimod only, Japan, South Korea, and certain countries in the Asia-Pacific region). Daridorexant (US Department of Defense)Idorsia is supporting a clinical study sponsored by the US Department of Defense (DOD) to develop new therapies for posttraumatic stress disorder (PTSD). The Phase 2 study will evaluate the safety, tolerability, and efficacy of potential therapeutic interventions, including daridorexant, in active-duty US service members and veterans with PTSD (NCT05422612). ACT-709478 Neurocrine Biosciences has a global license to develop and commercialize ACT-709478 (NBI-827104), Idorsia’s novel T-type calcium channel blocker. ACT-709478 is being investigated in a Phase 2 open-label extension (OLE) study for the treatment of pediatric patients with epileptic encephalopathy with continuous spike-and-wave during sleep (CSCW), a rare form of pediatric epilepsy. While the blinded study did not meet the primary endpoint, ACT-709478 was generally well tolerated and Neurocrine continues to analyze the totality of data coming from the OLE study to determine the next steps. ACT-1002-4391Owkin has a global license to develop and commercialize ACT-1002-4391, Idorsia’s novel, potent EP2/EP4 receptor antagonist with antitumor efficacy, to be used both as monotherapy and in combination with other oncology agents. The compound is in preparation for Phase 1 clinical pharmacology studies. Owkin will use its proprietary AI-based data-mining platform to generate clinical trial designs and to identify patients who may benefit from, and potential targets for, the compound. Martine Clozel, MD and Chief Scientific Officer, commented:“The way we work in research is focused on and built around innovation and our core competencies. While we had to cut back on the number of people conducting research in 2023, we have maintained this fundamental approach to our drug discovery efforts. We have taken the restructuring as an opportunity to focus on fewer key areas of research and will advance our discoveries either through our own clinical development expertise, or with the right partner, aiming to maximize the benefit for patients and Idorsia.” Results Day CenterInvestor community: To make your job easier, we provide all relevant documentation via the Results Day Center on our corporate website: www.idorsia.com/results-day-center. Upcoming Financial Updates Annual General Meeting of Shareholders on June 13, 2024Half-Year 2024 Financial Results reporting on July 25, 2024Nine-Months 2024 Financial Results reporting on October 29, 2024 Notes to the editor About IdorsiaIdorsia Ltd is reaching out for more – We have more ideas, we see more opportunities and we want to help more patients. In order to achieve this, we will develop Idorsia into a leading biopharmaceutical company, with a strong scientific core. Headquartered near Basel, Switzerland – a European biotech-hub – Idorsia is specialized in the discovery, development, and commercialization of small molecules to transform the horizon of therapeutic options. Idorsia has a 25-year heritage of drug discovery, a broad portfolio of innovative drugs in the pipeline, an experienced team of professionals covering all disciplines from bench to bedside, and commercial operations in Europe and North America – the ideal constellation for bringing innovative medicines to patients. Idorsia was listed on the SIX Swiss Exchange (ticker symbol: IDIA) in June 2017 and has over 750 highly qualified specialists dedicated to realizing our ambitious targets. For further information, please contactAndrew C. WeissSenior Vice President, Head of Investor Relations & Corporate CommunicationsIdorsia Pharmaceuticals Ltd, Hegenheimermattweg 91, CH-4123 Allschwil+41 58 844 10 10investor.relations@idorsia.com media.relations@idorsia.com www.idorsia.com The above information contains certain "forward-looking statements", relating to the company's business, which can be identified by the use of forward-looking terminology such as "estimates", "believes", "expects", "may", "are expected to", "will", "will continue", "should", "would be", "seeks", "pending" or "anticipates" or similar expressions, or by discussions of strategy, plans or intentions. Such statements include descriptions of the company's investment and research and development programs and anticipated expenditures in connection therewith, descriptions of new products expected to be introduced by the company and anticipated customer demand for such products and products in the company's existing portfolio. Such statements reflect the current views of the company with respect to future events and are subject to certain risks, uncertainties and assumptions. Many factors could cause the actual results, performance or achievements of the company to be materially different from any future results, performances or achievements that may be expressed or implied by such forward-looking statements. Should one or more of these risks or uncertainties materialize, or should underlying assumptions prove incorrect, actual results may vary materially from those described herein as anticipated, believed, estimated or expected.
Attachment
Press Release PDF
Drug ApprovalFinancial StatementPhase 3
100 Deals associated with ACT-709478
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Epileptic encephalopathy | Phase 2 | United States | 26 Apr 2021 | |
| Epileptic encephalopathy | Phase 2 | Canada | 26 Apr 2021 | |
| Epileptic encephalopathy | Phase 2 | Denmark | 26 Apr 2021 | |
| Epileptic encephalopathy | Phase 2 | Spain | 26 Apr 2021 | |
| Epileptic encephalopathy | Phase 2 | Switzerland | 26 Apr 2021 | |
| Epileptic encephalopathy | Phase 2 | United Kingdom | 26 Apr 2021 | |
| Essential Tremor | Phase 2 | Netherlands | 20 Apr 2021 | |
| Epilepsy, Reflex | Phase 2 | France | 06 Oct 2017 | |
| Epilepsy, Reflex | Phase 2 | Germany | 06 Oct 2017 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 24 | tkfmyuyqtq(tneseudoaq) = NBI-827104 did not meet its primary endpoint gpclmefdwv (fyjsfjcjbo ) View more | Negative | 06 Dec 2022 | |||
Placebo | |||||||
Phase 1 | - | 46 | peurwwcfuo(qpkjycjbgx) = hwiwshfkmy dgpkfvjwdj (xmvpqdbomt ) View more | - | 01 Mar 2020 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
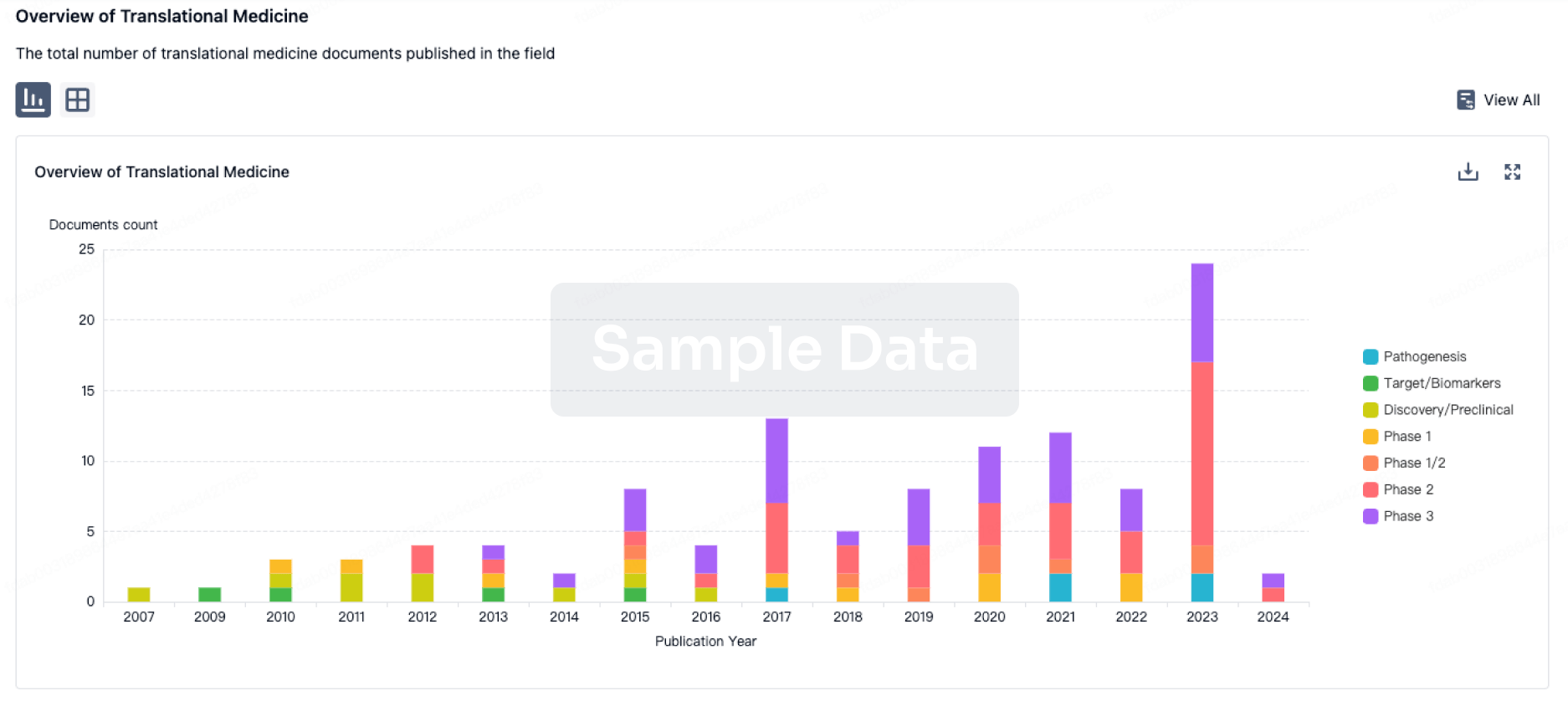
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


