Request Demo
Last update 20 Dec 2025
BST-02
Last update 20 Dec 2025
Overview
Basic Info
Drug Type TIL therapy |
Synonyms BST 02, BST02 |
Target- |
Action- |
Mechanism- |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
License Organization- |
Drug Highest PhasePhase 1 |
First Approval Date- |
RegulationFast Track (United States) |
Login to view timeline
Related
2
Clinical Trials associated with BST-02NCT06526832
A Phase 1, Dose-Escalation and Dose-Expansion Study Evaluating the Safety, Tolerability and Efficacy of BST02 in Treating Locally Advanced Liver Cancer
The goal of this clinical trial is to investigate the BST02, a tumor infiltrating lymphocyte product, collected from the locally advanced liver cancer subjects
The main questions it aims to answer are:
1. Safety and tolerability of BST02.
2. Preliminary efficacy of BST02. Participants will receive a cytoreductive surgery to collect samples for the manufacture of BST02.
Participants will receive BST02 infusion followed by IL-2 infusion. Blood samples will be collected for the analysis of PK and safety.
The main questions it aims to answer are:
1. Safety and tolerability of BST02.
2. Preliminary efficacy of BST02. Participants will receive a cytoreductive surgery to collect samples for the manufacture of BST02.
Participants will receive BST02 infusion followed by IL-2 infusion. Blood samples will be collected for the analysis of PK and safety.
Start Date04 Jul 2024 |
Sponsor / Collaborator |
NCT06173726
Investigator-initiated Phase I Exploratory Clinical Study of the Safety, Tolerability, and Efficacy of BST02 Injection in the Treatment of Locally Advanced/Metastatic Liver Cancer
This is an open, single-arm, investigator-initiated Phase I clinical trial to evaluate the safety, tolerability, and initial efficacy of BST02 injection in patients with locally advanced / metastatic liver cancer. This study includes a dose escalation study and a dose extension study, which will observe the effects of different IL-2 injection doses on the safety and efficacy of BST02.
After signing the informed consent, the subjects will roughly go through two periods: the main study period and the long-term follow-up period. The main study period includes screening period, treatment and safety observation period, and follow-up period
After signing the informed consent, the subjects will roughly go through two periods: the main study period and the long-term follow-up period. The main study period includes screening period, treatment and safety observation period, and follow-up period
Start Date05 Dec 2023 |
Sponsor / Collaborator  BIOSYNGEN PTE LTD BIOSYNGEN PTE LTD [+1] |
100 Clinical Results associated with BST-02
Login to view more data
100 Translational Medicine associated with BST-02
Login to view more data
100 Patents (Medical) associated with BST-02
Login to view more data
57
Literatures (Medical) associated with BST-0201 Dec 2023·Ultrasonics sonochemistry
Sonophotocatalytic water splitting by BaTiO3@SrTiO3 core shell nanowires
Article
Author: Shin, Taeho ; Vadivel, Sethumathavan ; Mohan, Harshavardhan
Sonophotocatalysis has garnered significant attention due to its potential to enhance advanced oxidation processes, particularly water splitting, by employing materials with combined sonocatalytic and photocatalytic properties. In this study, we synthesized and investigated core-shell BaTiO3@SrTiO3 nanowires (BST NWs) with varying Sr/Ba molar ratios (2.5:7.5, 5.0:5.0, 7.5:2.5 mM, denoted as BST-1, BST-2, and BST-3, respectively) as catalysts for hydrogen production through water splitting. The piezoelectric nanowires demonstrated hydrogen evolution via both sonocatalysis and photocatalysis. In the sonophotocatalysis process, the ultrasonic vibration induced mechanical forces on the BST nanowires, thereby establishing a built-in electric field. This built-in electric field facilitated the effective separation of photo-generated charge carriers and prolonged their lifetimes, leading to a synergistic enhancement of hydrogen evolution. The pristine BaTiO3 and SrTiO3 nanowires exhibited relatively low hydrogen evolution rates (HER) of 7.0 and 6.0 µmol·g-1min-1, respectively. In contrast, the core-shell nanowires exhibited a substantial improvement in the hydrogen evolution rate. The HER increased with the addition of Sr, and BST-1, BST-2, and BST-3 achieved HERs of 12.0, 13.5, and 18.0 µmol·g-1min-1, respectively. The superior performance of BST-3 nanowires can be attributed to their highest piezoelectric potential and largest surface area. Additionally, BST-3 nanowires demonstrated remarkable stability over multiple cycles, validating their practical applicability as efficient photocatalysts.
23 Feb 2022·JOURNAL OF VIROLOGY
Simian Immunodeficiency Virus SIVgsn-99CM71 Vpu Employs Different Amino Acids To Antagonize Human and Greater Spot-Nosed Monkey BST-2
Article
Author: Strebel, Klaus ; Yamaoka, Shoji ; Yoshida, Takeshi ; Yao, Weitong
Genes related to survival against life-threating pathogens are important determinants of natural selection in animal evolution. For instance, BST-2, a protein showing broad-spectrum antiviral activity, shows polymorphisms entailing different phenotypes even among primate species, suggesting that the
bst-2
gene of primates has been subject to strong selective pressure during evolution.
01 Jan 2022·Microbial pathogenesisQ3 · MEDICINE
APOBEC3, TRIM5α, and BST2 polymorphisms in healthy individuals of various populations with special references to its impact on HIV transmission
Q3 · MEDICINE
Review
Author: Nain, Sumitra ; Singh, Amita ; Arif Khan, Abdul ; Choudhari, Ranjana ; Maan, Harjeet Singh ; Aggarwal, Sumit ; Aggarwal, Shubham K ; Gupta, Vivek ; Verma, Sheetal ; Jadhav, Sushama ; Singh, HariOm
AIDS restriction genes (ARGs) like APOBEC3, TRIM5α, and BST2 can act as immunological detectors of the innate protective mechanism of the body. ARGs influence the course of viral pathogenesis and progression of the disease. The infection caused by different viruses including HIV activates the innate immune receptors leading to production of proinflammatory cytokines, interferons and signals that recruit and activate cells involved in the process of inflammation following induction of adaptive immunity. Differential expression of genes involved in viral infection decide the fate and subsequent susceptibility to infection and its clinical outcome. Nevertheless, comprehensive reports on the incidence of genetic polymorphism of APOBEC3s, TRIM5α, and BST-2 in the general population and its association with pathological conditions have not been described well. Therefore, the occurrence of APOBEC3, TRIM5α, and BST2 polymorphism in healthy individuals and its impact on HIV transmission was analyzed. We conducted an extensive search using the several databases including, EMBASE, PubMed (Medline), and Google Scholar. APOBEC3-D, -F, -G, and -H out of the seven human APOBEC3s, help in the control of viral infection. Amongst various restriction factors, TRIM5α and BST-2 also restrict the viral infection followed by the development of the disease. In the current review, a brief account of the polymorphism in the APOBEC3G, TRIM5α, and BST2 genes are explored among different populations along with the interaction of APOBEC3G with Vif protein. Furthermore, this review specifically focus on ARGs polymorphism (APOBEC3G, TRIM5α, and BST2) associated with HIV transmission.
7
News (Medical) associated with BST-0212 Aug 2025
Over the last ten years, cell-based immunotherapy has emerged as a promising approach for treating solid tumors. Several companies, including Iovance Biotherapeutics, Obsidian Therapeutics, Biosyngen, KSQ Therapeutics, and others, are currently engaged in the development and production of TIL therapies, which have the potential to significantly impact and enhance the TIL therapies market.
LAS VEGAS, Aug. 12, 2025 /PRNewswire/ -- DelveInsight's
Tumor-Infiltrating Lymphocyte Therapies Market Size, Target Population, Competitive Landscape & Market Forecast report includes a comprehensive understanding of current treatment practices, addressable patient population, which includes top indications such as melanoma, cervical cancer, non-small cell lung cancer (NSCLC), endometrial cancer, head and neck squamous cell carcinoma (HNSCC), colorectal cancer, and other solid tumors. The selected indications are based on approved therapies and ongoing pipeline activity. The report also provides insights into the emerging tumor-infiltrating lymphocyte therapies, market share of individual therapies, and current and forecasted market size from 2020 to 2034, segmented into 7MM.
Key Takeaways from the Tumor-Infiltrating Lymphocyte Therapies Market Report
As per DelveInsight's analysis, the total market size of tumor-infiltrating lymphocyte therapies in the 7MM is expected to surge significantly by 2034.
The report provides the total potential number of patients in the indications, such as
melanoma, cervical cancer, non-small cell lung cancer (NSCLC), endometrial cancer, head and neck squamous cell carcinoma (HNSCC), colorectal cancer, and other solid tumors.
Leading tumor-infiltrating lymphocyte therapies companies, such as
Obsidian Therapeutics, Biosyngen, KSQ Therapeutics, and others, are developing novel tumor-infiltrating lymphocyte therapies that can be available in the tumor-infiltrating lymphocyte therapies market in the coming years.
Some of the key tumor-infiltrating lymphocyte therapies in the pipeline include
OBX-115, BST02, KSQ-001 EX, KSQ-004 EX, and others.
In
June 2025, Iovance Biotherapeutics announced that the Journal of Clinical Oncology has published the final analysis from the Phase II C-144-01 clinical trial evaluating the individualized T cell therapy AMTAGVI in patients with advanced melanoma. These five-year follow-up results are being simultaneously presented during an oral session today at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting.
In
June 2025, Obsidian Therapeutics announced initial Phase I safety and efficacy data from the Phase I/II Agni-01 multicenter study of OBX-115 a summarized the data will be presented by the Director and Chief Administrative Officer of UofL Health – Brown Cancer Center/Oncology Service Line at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting.
In
April 2025, KSQ Therapeutics announced the first patient dosed in the Phase I/II clinical study of KSQ-004EX, a novel CRISPR-engineered Tumor Infiltrating Lymphocyte (eTIL) therapy. KSQ-004EX consists of eTIL in which the SOCS1 and Regnase-1 genes are inactivated by CRISPR/Cas9 gene editing, giving it the potential to be a best-in-class treatment for a variety of solid tumor indications. KSQ's CRISPRomics platform identified SOCS1 and Regnase-1 as key genes inhibiting the ability of TIL to eradicate solid tumors in preclinical models.
Discover which indication is expected to grab the major tumor-infiltrating lymphocyte therapies market share @
Tumor-Infiltrating Lymphocyte Therapies Market Report
Tumor-Infiltrating Lymphocyte Therapies Market Dynamics
The tumor-infiltrating lymphocyte therapies market is poised for transformative growth, driven by
increasing interest in personalized and cell-based immunotherapies for solid tumors. The market gained significant momentum following the U.S. FDA
approval of Iovance Biotherapeutics' AMTAGVI in 2024 for advanced melanoma, marking the
first regulatory validation of a TIL therapy and paving the way for wider clinical adoption and investor confidence.
A major driver of the market is the
growing unmet need in solid tumor treatment, where conventional immunotherapies like checkpoint inhibitors have limited efficacy for many patients. TIL therapies offer a more tailored solution, capable of overcoming tumor immune evasion by targeting multiple neoantigens.
Advances in cell manufacturing, automation, and closed-system bioprocessing are also contributing to improved scalability and reduced turnaround times, addressing one of the historic limitations of TILs, the complex and time-intensive production process.
The
competitive landscape is becoming increasingly dynamic, with numerous biotechnology firms and academic institutions entering the TIL space.
Strategic partnerships, technology licensing, and mergers & acquisitions are prevalent as players aim to build robust manufacturing capabilities and differentiate their platforms using gene editing, selection techniques, or combination regimens with checkpoint inhibitors.
However, the market faces several challenges, including
high production costs, patient-specific manufacturing logistics, and the
need for specialized infrastructure.
Reimbursement hurdles, regulatory complexities, and the
need for biomarker-based patient selection also represent significant barriers to widespread commercialization. Nonetheless,
continued progress in artificial intelligence for neoantigen identification, cryopreservation methods, and decentralized manufacturing models could mitigate many of these issues over time.
In conclusion, the TIL therapies market is at an inflection point, transitioning from early-stage development to commercial viability. The combination of strong clinical data, regulatory tailwinds, and technological innovation is expected to drive robust growth in the coming decade. As more TIL therapies move into late-stage trials and receive regulatory approval, their role in the broader oncology treatment landscape is likely to expand, especially for cancers with high unmet needs and limited therapeutic options.
Tumor-Infiltrating Lymphocyte Therapies Treatment Market
TIL therapies represent a new generation of personalized immunotherapy designed to leverage a patient's own immune system to combat solid tumors. This approach involves extracting TILs from a patient's tumor, expanding and modifying them outside the body, and then reinfusing them to stimulate a targeted immune response. After years of academic research, the field reached a significant milestone in 2024 with the FDA approval of AMTAGVI by Iovance Biotherapeutics for treating advanced melanoma.
AMTAGVI is an autologous T cell therapy derived from a patient's tumor, approved for adult patients with unresectable or metastatic melanoma who have previously received a PD-1 inhibitor. For those with a BRAF V600 mutation, prior treatment with a BRAF inhibitor, with or without a MEK inhibitor, is also required. Although its exact mechanism of action is not fully understood, AMTAGVI is believed to work by utilizing the patient's own TILs.
These TILs naturally develop in response to cancer and are capable of recognizing and attacking tumor-specific markers. However, as cancer progresses, the effectiveness of these native TILs diminishes. AMTAGVI seeks to restore and enhance its function by reintroducing activated TILs into the patient. The therapy is also being evaluated in late-stage clinical trials, both as a standalone treatment and in combination with pembrolizumab, for other cancers such as non-small cell lung cancer and cervical cancer.
Learn more about the tumor-infiltrating lymphocyte therapies @
Tumor-Infiltrating Lymphocyte Therapies Analysis
Key Emerging Tumor-Infiltrating Lymphocyte Therapies and Companies
TIL therapies have a pipeline, with candidates such as O
BX-115, BST02, KSQ-001 EX, and KSQ-004 EX in early Phase (I/II) trials targeting diverse indications like advanced gastric, gastroesophageal junction, and other solid tumors.
OBX‑115 is an advanced tumor-infiltrating lymphocyte (TIL) therapy developed using Obsidian's proprietary cytoDRIVE platform. This therapy involves genetically modifying TILs to express membrane-bound interleukin-15 (mbIL‑15), which enhances T cell proliferation, durability, and antitumor effectiveness—eliminating the need for high-dose IL‑2, a key source of toxicity in traditional TIL therapies.
The OBX‑115 TILs are engineered to produce mbIL‑15 linked to a drug-responsive domain, enabling controlled, dose-dependent increases in mbIL‑15 levels when an FDA-approved stabilizing agent, acetazolamide (ACZ), is administered. This approach further removes the requirement for IL‑2.
In September 2024, Obsidian Therapeutics announced that the U.S. FDA granted OBX‑115 Regenerative Medicine Advanced Therapy (RMAT) designation for treating patients with unresectable or metastatic melanoma that no longer responds to immune checkpoint inhibitors (ICIs).
The anticipated launch of these emerging therapies are poised to transform the tumor-infiltrating lymphocyte therapies market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the tumor-infiltrating lymphocyte therapies market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about tumor-infiltrating lymphocyte therapies clinical trials, visit @
Tumor-Infiltrating Lymphocyte Therapies Treatment
Tumor-Infiltrating Lymphocyte Therapies Overview
Tumor-infiltrating lymphocyte (TIL) therapy is an advanced type of adoptive cell therapy (ACT) that involves isolating naturally occurring, polyclonal T cells from within a patient's tumor microenvironment, expanding them outside the body, and reintroducing them after the patient undergoes non-myeloablative lymphodepletion. Unlike engineered approaches such as CAR-T or TCR therapies, TIL therapy leverages the natural tumor-targeting ability of the patient's own T cells, which are already primed to detect a broad range of tumor-specific neoantigens.
The strategy is based on the idea that these tumor-resident T cells can generate a more effective and biologically relevant immune response. Once infused back into the patient, the expanded TILs can survive, home in on tumor sites, and exert cytotoxic effects on cancer cells. This approach has shown lasting clinical responses, especially in immunologically active cancers like metastatic melanoma, cervical cancer, and non-small cell lung cancer (NSCLC), including in cases resistant to immune checkpoint inhibitors.
Tumor-Infiltrating Lymphocyte Therapies Epidemiology Segmentation
The tumor-infiltrating lymphocyte therapies market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM, segmented into:
Total Cases of Selected Indications for TIL Therapies
Total Eligible Patients of the Selected Indications of TIL Therapies
Total Treated Cases in Selected Indications for TIL Therapies
Scope of the
Tumor-Infiltrating Lymphocyte Therapies
Market Report
Tumor-Infiltrating Lymphocyte Therapies Therapeutic Assessment: Tumor-Infiltrating Lymphocyte Therapies' current marketed and emerging therapies
Tumor-Infiltrating Lymphocyte Therapies
Market Dynamics: Conjoint Analysis of Emerging Tumor-Infiltrating Lymphocyte Therapies Drugs
Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
Unmet Needs, KOL's views, Analyst's views, Tumor-Infiltrating Lymphocyte Therapies Market Access and Reimbursement
Discover more about tumor-infiltrating lymphocyte therapies in development @
Tumor-Infiltrating Lymphocyte Therapies Clinical Trials
Table of Contents
Related Reports
Non-Small Cell Lung Cancer Market
Non-Small Cell Lung Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NSCLC companies, including
AstraZeneca, Boehringer Ingelheim, Takeda, Johnson & Johnson Innovative Medicine, Eli Lilly and Company, Merck, Bristol-Myers Squibb, Roche, Shanghai Henlius Biotech, AbbVie, Daiichi Sankyo, Nuvation Bio, PDC*line Pharma, Moderna Therapeutics, Pfizer, GSK, Gilead Sciences, BieGene, Nuvalent, among others.
Melanoma Market
Melanoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key melanoma companies, including
Incyte Corporation, Merck Sharp & Dohme LLC, Pfizer, Bristol-Myers Squibb, Medarex, BioVex Limited, Amgen, IO Biotech, Celgene, among others.
Endometrial Cancer Market
Endometrial Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key endometrial cancer companies, including
GlaxoSmithKline, Merck & Co., AstraZeneca, Karyopharm Therapeutics, Evergreen Therapeutics, Incyte Corporation, among others.
Cervical Cancer Market
Cervical Cancer Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cervical cancer companies, including
Roche, AstraZeneca, Precigen, Puma Biotechnology, Iovance Biotherapeutics, Nykode Therapeutics, ISA Pharmaceuticals, Regeneron, Transgene, Daiichi Sankyo, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Contact Us
Shruti Thakur
[email protected]
+14699457679
Logo:
SOURCE DelveInsight Business Research, LLP
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
Phase 1ImmunotherapyASCODrug ApprovalCell Therapy
20 Sep 2024
BARCELONA, Spain, Sept. 20, 2024 /PRNewswire/ -- The highly anticipated
2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place from September 13 to 17 in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchers from around the globe, showcasing the latest advancements in the field and providing high-quality educational and networking opportunities for oncology professionals.
Continue Reading
Figure 1 BSGTIL antitumor efficacy
Biosyngen, an innovative biotechnology company specializing in immune cell therapies, has showcased its
groundbreaking technology on gene-modified, functionally enhanced tumor-infiltrating lymphocytes (TILs) derived from liver cancer biopsy samples.
BioSyngen has secured ten clinical trial approvals in China and the U.S. for its innovative fourth-generation oncology therapies. Currently,
our leading pipeline product, BRG01, is in the pivotal Phase II clinical trial stage for solid tumors. Additionally, the first patients have been enrolled in the Phase I trials for our other groundbreaking therapies, BST02 and BRL03, with completion of Phase I trials anticipated later this year.
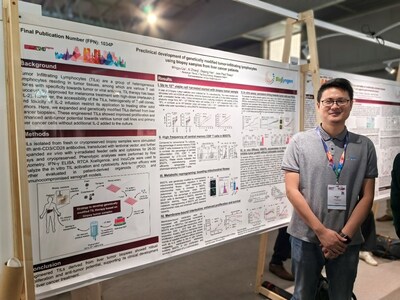
Abstract Title
Pre-clinical Development of Genetically Modified Tumor-infiltrating Lymphocytes using Biopsy Samples from Liver Cancer Patients
Abstract No.
1034P
Tumor-infiltrating lymphocytes (TILs) are heterogeneous lymphocyte populations within the tumor microenvironment that contains T cells capable of recognizing tumor- or virus-associated antigens.
In February of this year, the FDA approved the first TIL-based therapy used with IL-2 for advanced metastatic/recurrent melanoma. However, due to the variability in T cell infiltration between hot and cold tumors, differential abundance of antigen-specific T cells with robust immune response functionality, and the dependence of TIL anti-tumor efficacy on concomitant use of high-dose IL-2 combination therapy, the therapeutic applications of non-edited TILs are quite limited outside of melanoma.
To address these challenges,
Biosyngen has developed a proprietary platform for expanding TILs from biopsy samples, achieving production of 1011 cells within four weeks. Additionally, the company has established a stable gene modification platform that reprograms TIL metabolism, enhances TIL activity and sustained antitumor efficacy by expressing membrane anchor proteins. Anti-tumor efficacy has significantly enhanced (without IL-2 co-injection) and no obvious toxicity observed.
Biosyngen houses best-in-class proprietary TIL platform aiming to expand the clinical applications of TIL technology with following features:
Efficient Automated TILs Manufacturing System: Pioneering the use of tumor biopsy samples for TIL preparation with the ability to cryopreserve both tumor tissue and final products, overcoming logistical constraints.
Effective In Vitro Gene-engineering System: Employs viral vector technology for stable gene modification, maintaining high gene expression efficiency in TILs.
Enhanced In Vivo Expansion and Persistence: Increased proportion of central memory T cells (TCM) in the final product, leading to prolonged persistence.
Powerful Antitumor Efficacy: Demonstrates strong tumor-killing effects without the need for concomitant use of IL-2.
SOURCE Biosyngen
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
Gene TherapyPhase 1Phase 2Drug Approval
02 Feb 2024
Biosyngen, a leading pharmaceutical company, has announced that its groundbreaking product, BST02, has been granted Fast Track Designation (FTD) by the US Food and Drug Administration (FDA) for the treatment of all types of liver cancer, including hepatocellular carcinoma and cholangiocarcinoma.
Fast Track Designation is a special process designed to facilitate the development and expedite the review of drugs intended to treat serious or life-threatening conditions, addressing unmet medical needs. This designation is expected to accelerate the progression of clinical trials and streamline the registration of pharmaceutical products for marketing purposes.
BST02, the first TIL cell therapy drug for liver cancer to advance to the clinical stage globally, received FDA approval for Phase I/II clinical trials in October 2023. Additionally, it obtained approval from the Center for Drug Evaluation (CDE) of the China National Drug Administration in January 2024. This achievement underscores Biosyngen's commitment to pioneering cell therapy for cancer treatment.
Another Biosyngen product, BRG01, had previously been granted Fast Track Designation in July 2023, further highlighting the company's dedication to addressing critical medical needs.
Michelle Chen, co-founder and CEO of Biosyngen, expressed gratitude to the FDA for acknowledging BST02 as the company's fourth product. She reiterated Biosyngen's commitment to advancing global cancer cell therapy and providing innovative treatment solutions for diverse patient populations. Chen emphasized the company's dedication to utilizing advanced technology platforms to offer more effective and accessible treatment options globally.
BST02, a T-cell therapy based on the expansion of the patient's tumor-infiltrating lymphocytes, falls under the category of adoptive immune cell therapy technology. Offering promise for the treatment of all types of liver cancer, BST02 stands out with its cryopreserved form, overcoming distance constraints, and a reduced need for high doses of interleukin-2 compared to traditional TIL therapies.
Biosyngen remains dedicated to the research and development of innovative pharmaceutical products to address unmet clinical needs in oncology. The company looks forward to maintaining its focus on advancing research in this domain for the betterment of patients worldwide.

Fast TrackCell TherapyINDImmunotherapyClinical Study
100 Deals associated with BST-02
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Hepatocellular Carcinoma | Phase 1 | China | 05 Jul 2024 | |
| Locally Advanced Hepatocellular Carcinoma | Phase 1 | China | 04 Jul 2024 | |
| Locally Advanced Malignant Neoplasm | Phase 1 | China | 04 Jul 2024 | |
| Advanced Hepatocellular Carcinoma | IND Approval | China | 24 Jan 2024 | |
| Intrahepatic Cholangiocarcinoma | IND Approval | China | 24 Jan 2024 | |
| Liver Cancer | IND Approval | United States | 26 Oct 2023 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
NCT06526832 (ASCO2025) Manual | Phase 1 | 11 | BST02 5 × 10^9 viable cells+ IL-2 (cohort 1) | tasrerlyvb(qkbkcnupwa) = lefiqxuflx xdytdwsxct (ecxyldvwqz ) View more | Positive | 30 May 2025 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free