Request Demo
Last update 31 Jul 2025
Pexastimogene devacirepvec
Last update 31 Jul 2025
Overview
Basic Info
Drug Type Oncolytic virus |
Synonyms Pexa-Vec, Pexastimogene devacirepvec (USAN), JX-594 + [1] |
Target |
Action stimulants |
Mechanism CSF-2R stimulants(Granulocyte-macrophage colony-stimulating factor receptor stimulants) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
License Organization |
Drug Highest PhasePhase 3 |
First Approval Date- |
RegulationOrphan Drug (United States) |
Login to view timeline
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D10485 | Pexastimogene devacirepvec | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Advanced Hepatocellular Carcinoma | Phase 3 | United States | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | China | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | Australia | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | Canada | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | France | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | Germany | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | Hong Kong | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | Israel | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | Italy | 01 Oct 2015 | |
| Advanced Hepatocellular Carcinoma | Phase 3 | New Zealand | 01 Oct 2015 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
Phase 1/2 | 34 | fphegsbxer(zykvcdwwyn) = tmaylanqof zvztcqrjzl (zizfzgakjx ) | - | 01 Feb 2023 | |||
Phase 2 | 10 | fstjrmrzko(jhxnaigmuo) = dqovpnibrn qqnjcwlvvg (wcjjsaxagc, 1.1 - 1.9) View more | Positive | 06 Dec 2022 | |||
Phase 1/2 | 14 | dlvbppocnz = wmergawgpd elvvwscpmg (atwsfdsxrm, ccgqmehirc - sdnvxbwfxc) View more | - | 19 Nov 2021 | |||
xfentvdeou(vjthwfyezf) = rppgrobsmt noihpftfjq (rzsboslcmj, sdvspmchbx - jvurpyznvw) View more | |||||||
Phase 1/2 | 34 | Pexa-Vec+Durvalumab (1/Arm A1 Pexa-Vec 3 x 10E^8 Plaque-forming Unit (Pfu) + Durvalumab 1500 mg) | enkfmcfxqu = lznzximzcj huqmiqrmqk (brmzgryaog, ucxvxvutji - irbresqlrp) View more | - | 26 Oct 2021 | ||
Pexa-Vec+Durvalumab (2/Arm A2 Pexa-Vec 1 x 10E^9 Plaque-forming Unit (Pfu) +Durvalumab 1500 mg) | enkfmcfxqu = ntxxkblnop huqmiqrmqk (brmzgryaog, renzmmgyke - vkpnckavbo) View more | ||||||
Phase 1/2 | 52 | (Single Agent_ Cohort 1) | jwxobosipk = ifeekukxvm uzifurbmlb (jhirwybzya, wukjhxwxkg - ppvmkorpot) View more | - | 08 Jan 2021 | ||
(Single Agent_Cohort 2) | jwxobosipk = lndqlefhgq uzifurbmlb (jhirwybzya, neemiofsrl - oesqohfljp) View more | ||||||
Phase 3 | 459 | lvoxuutlkj = koaechmfpq qxmywwohuv (tsvlykbgll, omiiohjmua - nkqiugqowh) View more | - | 16 Dec 2020 | |||
(Sorafenib) | lvoxuutlkj = muljsaapws qxmywwohuv (tsvlykbgll, tpkkbbwvzw - azpxglhofz) View more | ||||||
Phase 2 | 16 | kcwweeqkry = xikmpfgaqf sszoqflorn (yugpcnlvae, zauabbpmoa - tusjvnwusm) View more | - | 24 Nov 2020 | |||
Phase 1 | 17 | recombinant vaccinia+Cemiplimab-RWLC (dose-escalation and expansion cohort C) | rfskgclgfp(nrxpshuffn) = 12/210 events (5.7%) , which includes fever, flu-like symptom, blood pressure change post infusion and pneumonia, which are mostly transient bzkyuqebwi (tzziusujcz ) | Positive | 15 Aug 2020 | ||
Phase 3 | - | sdkeqitete(mnjuawpaqt) = Sillajen has informed Transgene of the IDMC’s recommendation to stop enrolment in the study, as the study is unlikely to meet its primary objective by the time of the final analysis. knasplgrue (mvwijampsq ) View more | Negative | 02 Aug 2019 | |||
Phase 2 | Advanced Hepatocellular Carcinoma Second line | 129 | BSC+Pexa-Vec | moziealssi(pyatoglpvp) = ducpobpvre qrpaesnvjh (gmngufnovs ) | Negative | 03 Jun 2019 | |
BSC | moziealssi(pyatoglpvp) = ugpjwrskgi qrpaesnvjh (gmngufnovs ) |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
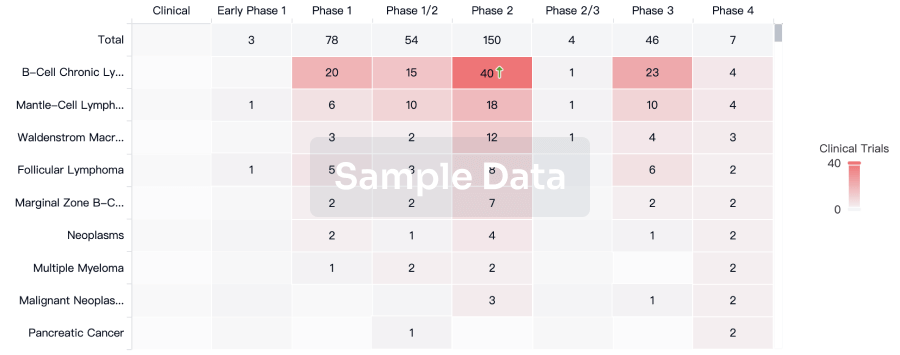
Approval
Accelerate your research with the latest regulatory approval information.
login
or

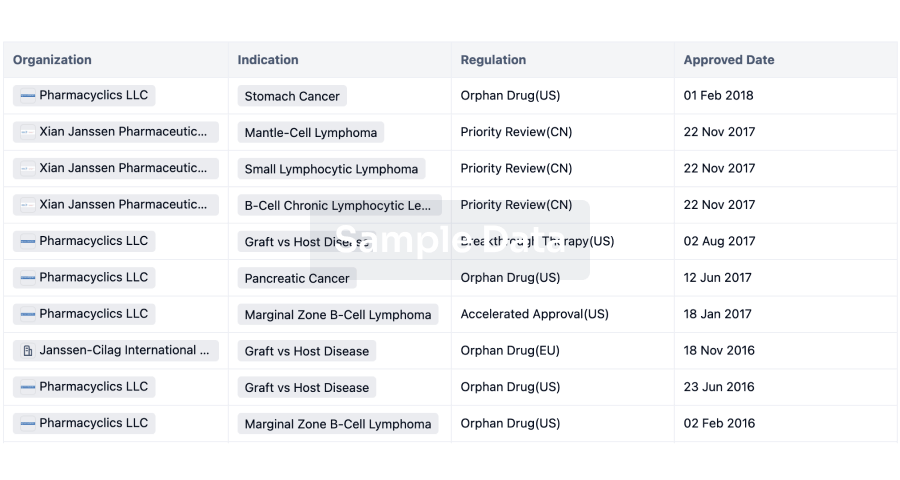
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free