Request Demo
Last update 21 Jun 2025
CC-90003
Last update 21 Jun 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms ERK inhibitor, CC 90003 |
Target |
Action inhibitors |
Mechanism ERK1 inhibitors(Extracellular-signal-regulated kinase1 inhibitors), ERK2 inhibitors(Extracellular-signal-regulated kinase 2 inhibitors) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization- |
Drug Highest PhaseDiscontinuedPhase 1 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Molecular FormulaC22H21F3N6O2 |
InChIKeyILUKRINUNLAVMH-UHFFFAOYSA-N |
CAS Registry1621999-82-3 |
Related
1
Clinical Trials associated with CC-90003NCT02313012
A Phase 1a Multicenter, Open-label Safety, Tolerability and Pharmacokinetic Study of CC-90003, a Selective Extracellular Signal-Regulated Kinase (ERK) Inhibitor, in Subjects With Locally-Advanced or Metastatic, Relapsed, or Refractory BRAF or RAS-Mutated Malignancies
The CC-90003-ST -001 trial is a first-in-man, open-label study in subjects with locally-advanced or wide spread cancers to determine if CC-90003 (an oral medication) can be adequately tolerated with minimal side effects.
Start Date05 Jan 2015 |
Sponsor / Collaborator |
100 Clinical Results associated with CC-90003
Login to view more data
100 Translational Medicine associated with CC-90003
Login to view more data
100 Patents (Medical) associated with CC-90003
Login to view more data
1,170
Literatures (Medical) associated with CC-9000301 Sep 2025·Neural Regeneration Research
Recombinant chitinase-3-like protein 1 alleviates learning and memory impairments via M2 microglia polarization in postoperative cognitive dysfunction mice.
Article
Author: Zhou, Yiming ; Ma, Zhengliang ; Gu, Xiaoping ; Han, Xue ; Xu, Jiyan ; Xu, Minhui ; Xia, Tianjiao ; Liu, Yujia ; Su, Yan
JOURNAL/nrgr/04.03/01300535-202509000-00032/figure1/v/2024-11-05T132919Z/r/image-tiff Postoperative cognitive dysfunction is a severe complication of the central nervous system that occurs after anesthesia and surgery, and has received attention for its high incidence and effect on the quality of life of patients. To date, there are no viable treatment options for postoperative cognitive dysfunction. The identification of postoperative cognitive dysfunction hub genes could provide new research directions and therapeutic targets for future research. To identify the signaling mechanisms contributing to postoperative cognitive dysfunction, we first conducted Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses of the Gene Expression Omnibus GSE95426 dataset, which consists of mRNAs and long non-coding RNAs differentially expressed in mouse hippocampus 3 days after tibial fracture. The dataset was enriched in genes associated with the biological process "regulation of immune cells," of which Chil1 was identified as a hub gene. Therefore, we investigated the contribution of chitinase-3-like protein 1 protein expression changes to postoperative cognitive dysfunction in the mouse model of tibial fracture surgery. Mice were intraperitoneally injected with vehicle or recombinant chitinase-3-like protein 1 24 hours post-surgery, and the injection groups were compared with untreated control mice for learning and memory capacities using the Y-maze and fear conditioning tests. In addition, protein expression levels of proinflammatory factors (interleukin-1β and inducible nitric oxide synthase), M2-type macrophage markers (CD206 and arginase-1), and cognition-related proteins (brain-derived neurotropic factor and phosphorylated NMDA receptor subunit NR2B) were measured in hippocampus by western blotting. Treatment with recombinant chitinase-3-like protein 1 prevented surgery-induced cognitive impairment, downregulated interleukin-1β and nducible nitric oxide synthase expression, and upregulated CD206, arginase-1, pNR2B, and brain-derived neurotropic factor expression compared with vehicle treatment. Intraperitoneal administration of the specific ERK inhibitor PD98059 diminished the effects of recombinant chitinase-3-like protein 1. Collectively, our findings suggest that recombinant chitinase-3-like protein 1 ameliorates surgery-induced cognitive decline by attenuating neuroinflammation via M2 microglial polarization in the hippocampus. Therefore, recombinant chitinase-3-like protein 1 may have therapeutic potential for postoperative cognitive dysfunction.
01 Jul 2025·CELLULAR SIGNALLING
The KSR1/MEK/ERK signaling pathway promotes the progression of intrauterine adhesions
Article
Author: Huang, Xiyue ; Sun, Congcong ; Yang, Xiao ; Wu, Shasha ; Zhang, Wenwen ; Wang, Jia ; Zhang, Lulu ; Chen, Qiuhong ; Huang, Jinglin ; Wu, Jiangling
Kinase suppressor of Ras 1 (KSR1) serves as a scaffold protein within the RAS-RAF pathway and plays a role in tumorigenesis, immune regulation, cell proliferation, and apoptosis. However, the specific role of KSR1 in the formation and progression of fibrotic diseases, such as intrauterine adhesions (IUA), remains unclear. This study aims to investigate KSR1 expression in IUA and the mechanisms underlying its role in promoting IUA progression. KSR1 was found to be significantly overexpressed in the endometrium of both IUA model rats and patients with IUA. KSR1 is positively involved in the regulation of proliferation, migration, and fibrosis (FN1, Collagen I, α-SMA) in immortalized human endometrial stromal cells (THESCs). Furthermore, KSR1 knockdown was observed to inhibit the fibrosis, proliferation, and migration of transforming growth factor-β1 (TGF-β1)-induced THESCs. Further studies demonstrated that the key proteins of the MEK/ERK signaling pathway, p-MEK1 and p-ERK1/2, were significantly overexpressed in the uterus of IUA rats. In vitro rescue experiments confirmed that the MEK/ERK pathway inhibitor U0126 (An ERK inhibitor) effectively suppressed the enhanced fibrosis, proliferation, and migration induced by KSR1 overexpression. In conclusion, this study demonstrates that KSR1 promotes IUA by enhancing proliferation, migration, and fibrosis of endometrial stromal cells via the MEK/ERK signaling pathway.
01 Jul 2025·JOURNAL OF HAZARDOUS MATERIALS
The tolerance mechanism of diarrhetic shellfish toxins mediated by the extracellular regulated protein kinase (ERK) pathway in the mussel Perna viridis
Article
Author: Mo, Yan-Hang ; Liu, Yang ; Lv, Jin-Jin ; Liu, Yu-Jie ; Zhang, Li ; Deng, Li-Yan ; Li, Hong-Ye ; Yang, Wei-Dong
Diarrheic shellfish toxins (DSTs) are a class of lipophilic algal toxins that accumulate excessively in bivalves following harmful algal blooms. Bivalves exhibit tolerance to DSTs, which make people ignore or underestimate the risk of DSTs, leading to the occurrence of seafood poisoning incidents. However, the tolerance mechanism remains unclear in bivalves. We investigated the role of extracellular-regulated protein kinase (ERK) in DSTs tolerance, and observed that the ERK inhibitor PD98059 exacerbated damage of DSTs to the digestive tubules. PD98059 induced the TUNEL fluorescence intensity, and caspase-3 activity inhibited by DSTs were restored to the control. PD98059 enhanced the fluorescence intensity of extracellular Ca-AM and increased the accumulation of esterified DSTs. Transcriptome analysis revealed that PD98059 affected the genes expression related to apoptosis, ABC transporters, and lipid metabolism. qPCR analysis demonstrated that PD98059 down-regulated the DSTs-induced iap and ABCC10 (p = 0.063), and up-regulated ABCB1-like1, ABCC1, ABCC1-like1, and ABCC9. Molecular docking suggested that ABCC10 exhibited high affinity for esterified okadaic acid. Overall, ERK plays a crucial role in DSTs tolerance by regulating the anti-apoptotic system and ABC transporters in bivalves. Our study is of great significance to understand the tolerance mechanism in bivalves and the safety risk caused by DSTs.
22
News (Medical) associated with CC-9000316 Apr 2025
Total sales growth of 11.6% at CER1, or 11.7% as reported, driven by all three therapeutic areas and including an increasing contribution from Iqirvo and Bylvay. Tovorafenib regulatory submission to EMA2 for pediatric low-grade glioma. Confirmation of full-year 2025 financial guidance.
PARIS, FRANCE, 16 April 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents sales for the first quarter of 2025.
“Ipsen has delivered a strong start to 2025, building further momentum in the transformation of our company,” commented David Loew, Chief Executive Officer, Ipsen. “We continued to execute on our strategy with strong top-line growth and pipeline progression. I am pleased to see the rapid build-up of our Rare Cholestatic Liver disease franchise with two innovate medicines for five indications. 2025 is set to be an important year for Ipsen, with multiple launches underway and several milestones expected across our portfolio.”
Full-year guidance
Ipsen is confirming financial guidance for full-year 2025:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in March 2025, a limited effect on total sales from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance includes expected negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe. It excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions.
Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in the European Union for advanced pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors (NETs). fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP). LANT3 (LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics.
Q1 pipeline progress
The regulatory filing for tovorafenib was accepted by EMA for review in the European Union, marking an important step forward in the development of this potential treatment for pediatric low-grade glioma and reinforcing Ipsen’s commitment to innovation in rare and difficult-to-treat cancers.
Ipsen also initiated the entry in Phase I of IPN01195, a RAF inhibitor, complementing IPN01194, an ERK inhibitor, and tovorafenib, two other assets targeting the MAPK pathway.
Group refinancing
Ipsen announced on March 19th the successful completion of its inaugural Rated Public Bond of €500 million with a coupon of 3.875%, maturing in March 2032. Following the disclosure of the Investment Grade ratings from S&P and Moody’s, this transaction was very well received and largely oversubscribed by a diversified and solid institutional investor base. This transaction is an important component of Ipsen’s refinancing plan which included the successful renewal of €1,5 billion syndicated Revolving Credit Facility, extending Ipsen’s debt maturity profile.
Conference call
A conference call and webcast for investors and analysts will begin today at 2pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its half-year results on July 31st, 2025.
Notes
All financial figures are in € millions (€m). The performance shown covers the three-month period to 31 March 2025 (Q1 2025, the quarter), compared to the three-month period to 31 March 2024 (Q1 2024), unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by internal and external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
InvestorsKhalid Deojee +33 6 69 09 12 96
MediaSally Bain +1 857 320 0517Anne Liontas +33 7 67 34 72 96
Disclaimers and/or forward-looking statements
The forward-looking statements, objectives and targets contained herein are based on Ipsen’s management strategy, current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated herein. All of the above risks could affect Ipsen’s future ability to achieve its financial targets, which were set assuming reasonable macroeconomic conditions based on the information available today. Use of the words ‘believes’, ‘anticipates’ and ‘expects’ and similar expressions are intended to identify forward-looking statements, including Ipsen’s expectations regarding future events, including regulatory filings and determinations. Moreover, the targets described in this document were prepared without taking into account external-growth assumptions and potential future acquisitions, which may alter these parameters. These objectives are based on data and assumptions regarded as reasonable by Ipsen. These targets depend on conditions or facts likely to happen in the future, and not exclusively on historical data. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably the fact that a promising medicine in early development phase or clinical trial may end up never being launched on the market or reaching its commercial targets, notably for regulatory or competition reasons. Ipsen must face or might face competition from generic medicine that might translate into a loss of market share. Furthermore, the research and development process involves several stages each of which involves the substantial risk that Ipsen may fail to achieve its objectives and be forced to abandon its efforts with regards to a medicine in which it has invested significant sums. Therefore, Ipsen cannot be certain that favorable results obtained during preclinical trials will be confirmed subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the safe and effective nature of the medicine concerned. There can be no guarantees a medicine will receive the necessary regulatory approvals or that the medicine will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Other risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and healthcare legislation; global trends toward healthcare cost containment; technological advances, new medicine and patents attained by competitors; challenges inherent in new-medicine development, including obtaining regulatory approval; Ipsen’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Ipsen’s patents and other protections for innovative medicines; and the exposure to litigation, including patent litigation, and/or regulatory actions. Ipsen also depends on third parties to develop and market some of its medicines which could potentially generate substantial royalties; these partners could behave in such ways which could cause damage to Ipsen’s activities and financial results. Ipsen cannot be certain that its partners will fulfil their obligations. It might be unable to obtain any benefit from those agreements. A default by any of Ipsen’s partners could generate lower revenues than expected. Such situations could have a negative impact on Ipsen’s business, financial position or performance. Ipsen expressly disclaims any obligation or undertaking to update or revise any forward-looking statements, targets or estimates contained in this press release to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based, unless so required by applicable law. Ipsen’s business is subject to the risk factors outlined in its registration documents filed with the French Autorité des Marchés Financiers. The risks and uncertainties set out are not exhaustive and the reader is advised to refer to Ipsen’s latest Universal Registration Document, available on ipsen.com.
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 EMA: European Medicines Agency 3 Long-acting neurotoxin
Attachment
Ipsen PR_Q1 2025_15042025
Total sales growth of 11.6% at CER1, or 11.7% as reported, driven by all three therapeutic areas and including an increasing contribution from Iqirvo and Bylvay. Tovorafenib regulatory submission to EMA2 for pediatric low-grade glioma. Confirmation of full-year 2025 financial guidance.
PARIS, FRANCE, 16 April 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents sales for the first quarter of 2025.
“Ipsen has delivered a strong start to 2025, building further momentum in the transformation of our company,” commented David Loew, Chief Executive Officer, Ipsen. “We continued to execute on our strategy with strong top-line growth and pipeline progression. I am pleased to see the rapid build-up of our Rare Cholestatic Liver disease franchise with two innovate medicines for five indications. 2025 is set to be an important year for Ipsen, with multiple launches underway and several milestones expected across our portfolio.”
Full-year guidance
Ipsen is confirming financial guidance for full-year 2025:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in March 2025, a limited effect on total sales from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance includes expected negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe. It excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions.
Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in the European Union for advanced pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors (NETs). fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP). LANT3 (LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics.
Q1 pipeline progress
The regulatory filing for tovorafenib was accepted by EMA for review in the European Union, marking an important step forward in the development of this potential treatment for pediatric low-grade glioma and reinforcing Ipsen’s commitment to innovation in rare and difficult-to-treat cancers.
Ipsen also initiated the entry in Phase I of IPN01195, a RAF inhibitor, complementing IPN01194, an ERK inhibitor, and tovorafenib, two other assets targeting the MAPK pathway.
Group refinancing
Ipsen announced on March 19th the successful completion of its inaugural Rated Public Bond of €500 million with a coupon of 3.875%, maturing in March 2032. Following the disclosure of the Investment Grade ratings from S&P and Moody’s, this transaction was very well received and largely oversubscribed by a diversified and solid institutional investor base. This transaction is an important component of Ipsen’s refinancing plan which included the successful renewal of €1,5 billion syndicated Revolving Credit Facility, extending Ipsen’s debt maturity profile.
Conference call
A conference call and webcast for investors and analysts will begin today at 2pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its half-year results on July 31st, 2025.
Notes
All financial figures are in € millions (€m). The performance shown covers the three-month period to 31 March 2025 (Q1 2025, the quarter), compared to the three-month period to 31 March 2024 (Q1 2024), unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by internal and external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
InvestorsKhalid Deojee +33 6 69 09 12 96
MediaSally Bain +1 857 320 0517Anne Liontas +33 7 67 34 72 96
Disclaimers and/or forward-looking statements
The forward-looking statements, objectives and targets contained herein are based on Ipsen’s management strategy, current views and assumptions. Such statements involve known and unknown risks and uncertainties that may cause actual results, performance or events to differ materially from those anticipated herein. All of the above risks could affect Ipsen’s future ability to achieve its financial targets, which were set assuming reasonable macroeconomic conditions based on the information available today. Use of the words ‘believes’, ‘anticipates’ and ‘expects’ and similar expressions are intended to identify forward-looking statements, including Ipsen’s expectations regarding future events, including regulatory filings and determinations. Moreover, the targets described in this document were prepared without taking into account external-growth assumptions and potential future acquisitions, which may alter these parameters. These objectives are based on data and assumptions regarded as reasonable by Ipsen. These targets depend on conditions or facts likely to happen in the future, and not exclusively on historical data. Actual results may depart significantly from these targets given the occurrence of certain risks and uncertainties, notably the fact that a promising medicine in early development phase or clinical trial may end up never being launched on the market or reaching its commercial targets, notably for regulatory or competition reasons. Ipsen must face or might face competition from generic medicine that might translate into a loss of market share. Furthermore, the research and development process involves several stages each of which involves the substantial risk that Ipsen may fail to achieve its objectives and be forced to abandon its efforts with regards to a medicine in which it has invested significant sums. Therefore, Ipsen cannot be certain that favorable results obtained during preclinical trials will be confirmed subsequently during clinical trials, or that the results of clinical trials will be sufficient to demonstrate the safe and effective nature of the medicine concerned. There can be no guarantees a medicine will receive the necessary regulatory approvals or that the medicine will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements. Other risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and healthcare legislation; global trends toward healthcare cost containment; technological advances, new medicine and patents attained by competitors; challenges inherent in new-medicine development, including obtaining regulatory approval; Ipsen’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of Ipsen’s patents and other protections for innovative medicines; and the exposure to litigation, including patent litigation, and/or regulatory actions. Ipsen also depends on third parties to develop and market some of its medicines which could potentially generate substantial royalties; these partners could behave in such ways which could cause damage to Ipsen’s activities and financial results. Ipsen cannot be certain that its partners will fulfil their obligations. It might be unable to obtain any benefit from those agreements. A default by any of Ipsen’s partners could generate lower revenues than expected. Such situations could have a negative impact on Ipsen’s business, financial position or performance. Ipsen expressly disclaims any obligation or undertaking to update or revise any forward-looking statements, targets or estimates contained in this press release to reflect any change in events, conditions, assumptions or circumstances on which any such statements are based, unless so required by applicable law. Ipsen’s business is subject to the risk factors outlined in its registration documents filed with the French Autorité des Marchés Financiers. The risks and uncertainties set out are not exhaustive and the reader is advised to refer to Ipsen’s latest Universal Registration Document, available on ipsen.com.
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 EMA: European Medicines Agency 3 Long-acting neurotoxin
Attachment
Ipsen PR_Q1 2025_15042025
Phase 2Phase 1Financial Statement
13 Feb 2025
FY 2024 total sales growth of 9.9% at CER1, or 8.7% as reported, with growth driven by strong performance across all therapeutic areas, including a 67.4% increase in the Rare Diseases portfolio, 9.2% in Neuroscience, and 7.3% in Oncology; Somatuline® (lanreotide) sales grew by 5.6%, while all other products, excluding Somatuline, achieved double-digit sales growth at 12.2% FY 2024 core operating income of €1,109m, growing 10.8% as reported, with core operating margin of 32.6% of total sales Continued pipeline expansion in 2024, with significant regulatory approvals, addition of several preclinical therapies with global rights and innovative modalities, and a late-stage asset Four key regulatory and clinical milestones expected in 2025, including the Proof-of-Concept data readout for the Long-Acting Neurotoxin (LANT) Financial guidance2 for 2025 including total sales growth greater than 5.0%3 at CER, and core operating margin greater than 30.0% of total sales, based on accelerated sales growth of the ex-Somatuline portfolio and assuming negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe
PARIS, FRANCE, 13 February 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents its financial results for the full year 2024.
“Ipsen delivered solid results and advanced its pipeline in 2024, laying a strong foundation for sustained growth,” said David Loew, Chief Executive Officer, Ipsen. “With the successful global rollout of Iqirvo and Bylvay, and the U.S. launch of Onivyde, alongside multiple business development deals adding several innovative assets, we are well positioned to execute our strategic roadmap. This year, we look forward to achieving key milestones, including the first data readout for the Long-Acting Neurotoxin (LANT), and further expand and progress our pipeline across all three therapeutic areas to bring promising new medicines to patients.”
Pipeline Progress
Significant regulatory milestones were achieved in 2024, including FDA approval of Onivyde® (irinotecan) for first-line pancreatic ductal adenocarcinoma (PDAC), along with accelerated U.S. approval and European approval for Iqirvo® (elafibranor), respectively. Additionally, Kayfanda® (odevixibat) was approved for Alagille syndrome (ALGS) in the E.U.
The company also opted-in for the CABINET Phase III study of Cabometyx® (cabozantinib) in patients with advanced neuroendocrine tumors (NETs), with study results presented at the 2024 European Society for Medical Oncology (ESMO) Congress and published in the New England Journal of Medicine.
An IND application was filed for IPN01194, an ERK inhibitor, advancing the potential medicine into clinical development with a Phase I/IIa trial in advanced solid tumors.
Ipsen improved further the depth and breadth of its pipeline by adding five preclinical innovative therapies with global rights and new modalities, and an ex-U.S. licensing agreement with DayOne Biopharmaceuticals for the late-stage oncology asset tovorafenib, an oral RAF inhibitor for pediatric low-grade glioma.
Two global licensing agreements for Antibody Drug Conjugate (ADC) in oncology with Sutro Biopharma and Foreseen Biotechnology were signed. An extension of the oncology partnership with Marengo Therapeutics to include TriSTAR, a next-generation precision T-cell engager was completed, as well as more recently, in the fourth quarter, a global licensing agreement with Biomunex for a preclinical novel T-cell engager (TCE). A collaboration with Skyhawk Therapeutics to develop RNA-modulating small molecules for rare neurological diseases was also signed during the year.
Ipsen executed several divestments in 2024, including the sale of Increlex® (mecasermin injection) to Eton Pharmaceuticals and the sale of its rare pediatric disease Priority Review Voucher.
Environmental, Social and Governance
Ipsen took important steps in 2024 in delivering its ambitious sustainability strategy. The company continued to integrate sustainability across its operations. From reducing its environmental footprint to advancing patient access and fostering a strong workplace culture, the company increased its commitment to driving progress for patients, employees, communities, and the planet.
Our sustainability efforts were recognized across multiple environment initiatives. The company achieved a 45% reduction in Scopes 1 & 2 greenhouse gas emissions and a 25% reduction in Scope 3, fully in line with its 2030 targets (versus 2019 baseline). Significant efforts were made to engage suppliers and third parties in Ipsen’s sustainability roadmap including the first-ever “Ipsen Supplier Sustainability Day”. Following an intensive transformation project, 99.8% of Ipsen’s global electricity now comes from renewable sources. Through the Fleet for Future project, the company continues to advance sustainable transportation, with 43% of its total company’s fleet now electric vehicles as of 2024.
We remain committed to gender balance in leadership, with women now representing 55% of the Global Leadership Team.
2025 Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in Europe for advanced neuroendocrine tumors (NETs), including pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors Tovorafenib (FIREFLY-1 trial) – Regulatory submission in Europe for pediatric low-grade glioma Fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP) LANT88(LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics
These milestones reinforce Ipsen’s commitment to advancing innovative therapies and expanding treatment options for patients worldwide.
2025 Financial Guidance
Ipsen has set for FY 2025 the following financial guidance, which excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in January 2025, a favorable effect on total sales of around 1% from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance on total sales and core operating margin is based on accelerated sales growth of the ex-Somatuline portfolio and assumes negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe.
Consolidated financial statements
The Board of Directors approved the consolidated financial statements on 12 February 2025. The consolidated financial statements have been audited and the Statutory Auditors’ report is in the process of being published. Ipsen’s comprehensive audited financial statements will be available in due course on ipsen.com (regulated-information section).
Conference call
A conference call and webcast for investors and analysts will begin today at 1pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its Q1 2025 sales on April 16th, 2025.
Notes
All financial figures are in € millions (€m), unless otherwise noted. The performance shown in this announcement covers the twelve-month period to 31 December 2024 (FY 2024) and the three-month period to 31 December 2024 (Q4 2024), compared to the twelve-month period to 31 December 2023 (FY 2023) and the three-month period to 31 December 2023 (Q4 2023), respectively, unless stated otherwise. The commentary is based on the performance in FY 2024, unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
Investors
Media
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 Excluding any impact from potential late-stage (Phase III clinical development or later) external-innovation transactions.3 Based on the average level of exchange rates in Jan 2024, a favorable effect on total sales of about 1% from currencies is expected. 4 Extract of consolidated results. The Company’s auditors performed a limited review of the condensed consolidated financial statements.5 Including an impairment loss of €279m (or €2,33 /share) related to Sohonos, reflecting lower revised sales following lower patient uptake.6 Dividend related to the current financial year to be paid the following year.7 Decided by the Ipsen S.A. Board of Directors and to be proposed at the annual shareholders’ meeting on 21 May 2025.
8 Long-acting neurotoxin
Attachment
Ipsen PR_FY 2024 – Results announcement_13022025
FY 2024 total sales growth of 9.9% at CER1, or 8.7% as reported, with growth driven by strong performance across all therapeutic areas, including a 67.4% increase in the Rare Diseases portfolio, 9.2% in Neuroscience, and 7.3% in Oncology; Somatuline® (lanreotide) sales grew by 5.6%, while all other products, excluding Somatuline, achieved double-digit sales growth at 12.2% FY 2024 core operating income of €1,109m, growing 10.8% as reported, with core operating margin of 32.6% of total sales Continued pipeline expansion in 2024, with significant regulatory approvals, addition of several preclinical therapies with global rights and innovative modalities, and a late-stage asset Four key regulatory and clinical milestones expected in 2025, including the Proof-of-Concept data readout for the Long-Acting Neurotoxin (LANT) Financial guidance2 for 2025 including total sales growth greater than 5.0%3 at CER, and core operating margin greater than 30.0% of total sales, based on accelerated sales growth of the ex-Somatuline portfolio and assuming negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe
PARIS, FRANCE, 13 February 2025 – Ipsen (Euronext: IPN; ADR: IPSEY), a global specialty-care biopharmaceutical company, today presents its financial results for the full year 2024.
“Ipsen delivered solid results and advanced its pipeline in 2024, laying a strong foundation for sustained growth,” said David Loew, Chief Executive Officer, Ipsen. “With the successful global rollout of Iqirvo and Bylvay, and the U.S. launch of Onivyde, alongside multiple business development deals adding several innovative assets, we are well positioned to execute our strategic roadmap. This year, we look forward to achieving key milestones, including the first data readout for the Long-Acting Neurotoxin (LANT), and further expand and progress our pipeline across all three therapeutic areas to bring promising new medicines to patients.”
Pipeline Progress
Significant regulatory milestones were achieved in 2024, including FDA approval of Onivyde® (irinotecan) for first-line pancreatic ductal adenocarcinoma (PDAC), along with accelerated U.S. approval and European approval for Iqirvo® (elafibranor), respectively. Additionally, Kayfanda® (odevixibat) was approved for Alagille syndrome (ALGS) in the E.U.
The company also opted-in for the CABINET Phase III study of Cabometyx® (cabozantinib) in patients with advanced neuroendocrine tumors (NETs), with study results presented at the 2024 European Society for Medical Oncology (ESMO) Congress and published in the New England Journal of Medicine.
An IND application was filed for IPN01194, an ERK inhibitor, advancing the potential medicine into clinical development with a Phase I/IIa trial in advanced solid tumors.
Ipsen improved further the depth and breadth of its pipeline by adding five preclinical innovative therapies with global rights and new modalities, and an ex-U.S. licensing agreement with DayOne Biopharmaceuticals for the late-stage oncology asset tovorafenib, an oral RAF inhibitor for pediatric low-grade glioma.
Two global licensing agreements for Antibody Drug Conjugate (ADC) in oncology with Sutro Biopharma and Foreseen Biotechnology were signed. An extension of the oncology partnership with Marengo Therapeutics to include TriSTAR, a next-generation precision T-cell engager was completed, as well as more recently, in the fourth quarter, a global licensing agreement with Biomunex for a preclinical novel T-cell engager (TCE). A collaboration with Skyhawk Therapeutics to develop RNA-modulating small molecules for rare neurological diseases was also signed during the year.
Ipsen executed several divestments in 2024, including the sale of Increlex® (mecasermin injection) to Eton Pharmaceuticals and the sale of its rare pediatric disease Priority Review Voucher.
Environmental, Social and Governance
Ipsen took important steps in 2024 in delivering its ambitious sustainability strategy. The company continued to integrate sustainability across its operations. From reducing its environmental footprint to advancing patient access and fostering a strong workplace culture, the company increased its commitment to driving progress for patients, employees, communities, and the planet.
Our sustainability efforts were recognized across multiple environment initiatives. The company achieved a 45% reduction in Scopes 1 & 2 greenhouse gas emissions and a 25% reduction in Scope 3, fully in line with its 2030 targets (versus 2019 baseline). Significant efforts were made to engage suppliers and third parties in Ipsen’s sustainability roadmap including the first-ever “Ipsen Supplier Sustainability Day”. Following an intensive transformation project, 99.8% of Ipsen’s global electricity now comes from renewable sources. Through the Fleet for Future project, the company continues to advance sustainable transportation, with 43% of its total company’s fleet now electric vehicles as of 2024.
We remain committed to gender balance in leadership, with women now representing 55% of the Global Leadership Team.
2025 Upcoming Milestones
Ipsen anticipates several key milestones across its portfolio in 2025, including:
Cabometyx (CABINET trial) – Regulatory decision in Europe for advanced neuroendocrine tumors (NETs), including pancreatic (pNETs) and extra-pancreatic (epNETs) neuroendocrine tumors Tovorafenib (FIREFLY-1 trial) – Regulatory submission in Europe for pediatric low-grade glioma Fidrisertib (FALKON trial) – Readout of the pivotal Phase IIb trial in fibrodysplasia ossificans progressiva (FOP) LANT88(LANTIC trial) – Proof-of-concept data readout, evaluating its potential in aesthetics
These milestones reinforce Ipsen’s commitment to advancing innovative therapies and expanding treatment options for patients worldwide.
2025 Financial Guidance
Ipsen has set for FY 2025 the following financial guidance, which excludes any impact from potential late-stage (Phase III clinical development or later) business development transactions:
Total sales growth greater than 5.0%, at constant currency. Based on the average level of exchange rates in January 2025, a favorable effect on total sales of around 1% from currencies is expected. Core operating margin greater than 30.0% of total sales, which includes additional R&D expenses from anticipated early and mid-stage external-innovation opportunities.
Guidance on total sales and core operating margin is based on accelerated sales growth of the ex-Somatuline portfolio and assumes negative impact on Somatuline sales due to increased generic competition in the U.S. and Europe.
Consolidated financial statements
The Board of Directors approved the consolidated financial statements on 12 February 2025. The consolidated financial statements have been audited and the Statutory Auditors’ report is in the process of being published. Ipsen’s comprehensive audited financial statements will be available in due course on ipsen.com (regulated-information section).
Conference call
A conference call and webcast for investors and analysts will begin today at 1pm CET. Participants can access the call and its details by registering here; webcast details can be found here.
Calendar
Ipsen intends to publish its Q1 2025 sales on April 16th, 2025.
Notes
All financial figures are in € millions (€m), unless otherwise noted. The performance shown in this announcement covers the twelve-month period to 31 December 2024 (FY 2024) and the three-month period to 31 December 2024 (Q4 2024), compared to the twelve-month period to 31 December 2023 (FY 2023) and the three-month period to 31 December 2023 (Q4 2023), respectively, unless stated otherwise. The commentary is based on the performance in FY 2024, unless stated otherwise.
About Ipsen
We are a global biopharmaceutical company with a focus on bringing transformative medicines to patients in three therapeutic areas: Oncology, Rare Disease and Neuroscience.
Our pipeline is fueled by external innovation and supported by nearly 100 years of development experience and global hubs in the U.S., France and the U.K. Our teams in more than 40 countries and our partnerships around the world enable us to bring medicines to patients in more than 100 countries.
Ipsen is listed in Paris (Euronext: IPN) and in the U.S. through a Sponsored Level I American Depositary Receipt program (ADR: IPSEY). For more information, visit ipsen.com.
Ipsen contacts
Investors
Media
1 At constant exchange rates (CER), which exclude any foreign-exchange impact by recalculating the performance for the relevant period by applying the exchange rates used for the prior period.2 Excluding any impact from potential late-stage (Phase III clinical development or later) external-innovation transactions.3 Based on the average level of exchange rates in Jan 2024, a favorable effect on total sales of about 1% from currencies is expected. 4 Extract of consolidated results. The Company’s auditors performed a limited review of the condensed consolidated financial statements.5 Including an impairment loss of €279m (or €2,33 /share) related to Sohonos, reflecting lower revised sales following lower patient uptake.6 Dividend related to the current financial year to be paid the following year.7 Decided by the Ipsen S.A. Board of Directors and to be proposed at the annual shareholders’ meeting on 21 May 2025.
8 Long-acting neurotoxin
Attachment
Ipsen PR_FY 2024 – Results announcement_13022025
Phase 2Drug ApprovalLicense out/inPhase 3
17 May 2024
Most of the affected roles at Erasca are located in drug discovery functions or deprioritized programs, the company said.
Erasca is spending a combined $22.5 million upfront to import fresh preclinical KRAS and molecular glue assets while clearing space in its own pipeline and laying off 18% of its employees in the process.
The San Diego-based biotech, which focuses on treatments for cancers driven by the RAS/MAPK pathway, is paying Medshine Discovery $10 million upfront for the worldwide rights to the KRAS inhibitor ERAS-4001. Erasca described the candidate as having “potential to provide an improved therapeutic window relative to RAS inhibitors and prevent KRAS wildtype-mediated resistance relative to mutant-selective approaches.”
Medshine will be in line for up to $160 million in milestone payments as well as a low single-digit percentage of the royalties should the drug make it to market.
To make room for ERAS-4001—and working on the assumption that Medshine will also be providing some ERAS-4 molecules as backup compounds—Erasca will call time on its internal pan-KRAS program. Erasca had already amended this program a year ago, when it blamed the “increasingly competitive landscape” for its decision to shelve a KRAS G12C inhibitor despite believing that the candidate was potentially differentiated.
It's not the only licensing deal Erasca announced yesterday evening. The company is also handing over $12.5 million to Joyo Pharmatech for the ex-China rights to a pan-RAS molecular glue called ERAS-0015. The candidate, which has now entered human trials, has demonstrated “5- to 10-fold greater in vitro and in vivo potency and favorable absorption, distribution, metabolism, and excretion properties and pharmacokinetic properties in multiple animal species,” Erasca explained in the release.
As part of the licensing agreement, China’s Joyo will also be in line for up to $176.5 million in milestone payments.
“We’re thrilled to add ERAS-0015 and ERAS-4001 to our pipeline,” Erasca CEO Jonathan Lim, M.D., said in the release. “Over the long term, we have a unique opportunity to combine these two best-in-class molecules with distinct and complementary RAS inhibitory mechanisms to ‘clamp’ RAS and shut down MAPK signaling for the benefit of patients with these common RAS mutations.”
In addition to its KRAS work, Erasca will be deprioritizing two other programs to ensure it has space for both ERAS-4001 and ERAS-0015. One of these is ERAS-007, an ERK inhibitor that was in a phase 1b trial in combination with Braftovi and Erbitux in EC-naïve patients with BRAF-mutant colorectal cancer. Clinical efficacy data to date “do not support continued evaluation,” the biotech explained.
The final amendment to its R&D strategy affects ERAS-801, a central nervous system-penetrant EGFR inhibitor being evaluated for recurrent glioblastoma. The biotech said that “due to the desire to focus internal resources” on its pan-RAF inhibitor naporafenib—which is due to enter a phase 3 melanoma trial in the coming weeks—as well as its broader RAS-targeting franchise, “Erasca is exploring further advancement of ERAS-801 via select investigator-sponsored trial(s).”
These pipeline changes will be accompanied by a workforce reduction that will see 18% of staff heading for the exits, with most of the affected roles located in drug discovery functions or the deprioritized programs.
“Erasca is deeply committed to easing this transition for its impacted colleagues and will offer comprehensive severance packages and career transition services,” the biotech added in the release.
“With the in-licensing of these RAS-targeting programs, we have made the difficult but necessary decision to deprioritize or externalize resourcing of our pipeline (ERAS-007, ERAS-801, and ERAS-4),” Lim explained. “This change has unfortunately impacted certain team members. We believe that further focusing our resources will allow us to advance the programs with the highest probability of success and largest potential for patient impact.”

Phase 1License out/in
100 Deals associated with CC-90003
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Locally Advanced Malignant Solid Neoplasm | Phase 1 | United States | 05 Jan 2015 | |
| Locally Advanced Malignant Solid Neoplasm | Phase 1 | Australia | 05 Jan 2015 |
Login to view more data
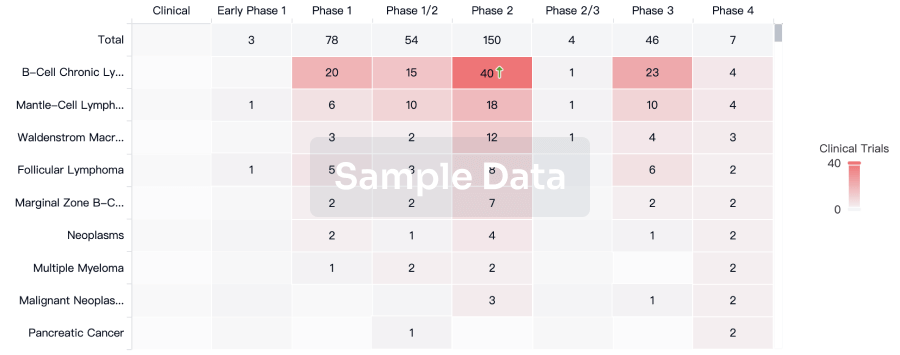
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 1 | 19 | jdubjpppxj(abulsdozwc) = vdrxbpvejp exhvqtzsax (wokkbnlsjy ) | Negative | 30 May 2017 |
Login to view more data
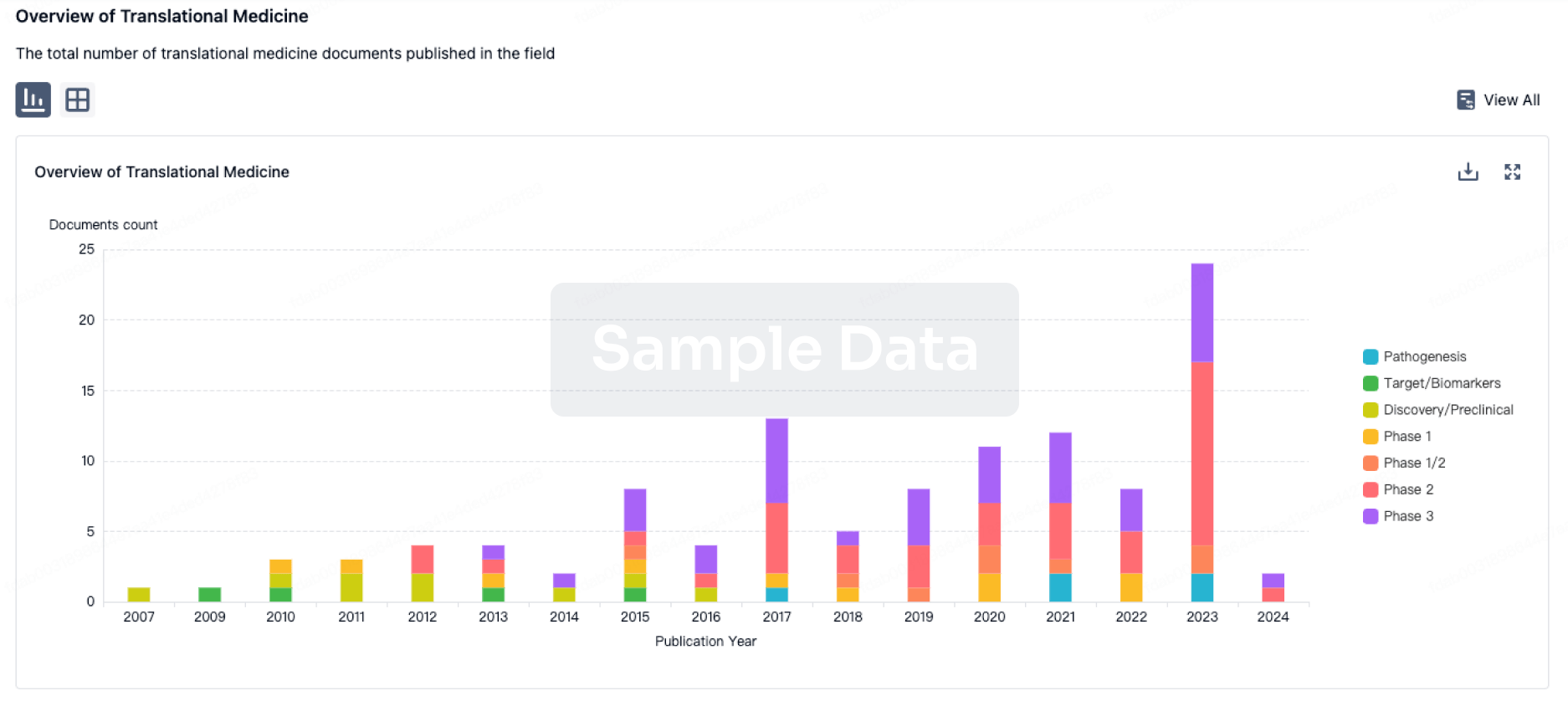
Translational Medicine
Boost your research with our translational medicine data.
login
or
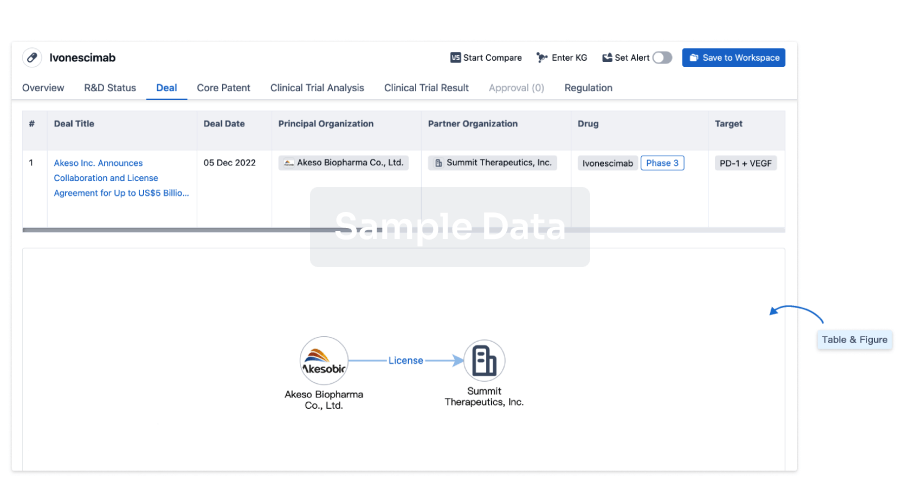
Deal
Boost your decision using our deal data.
login
or
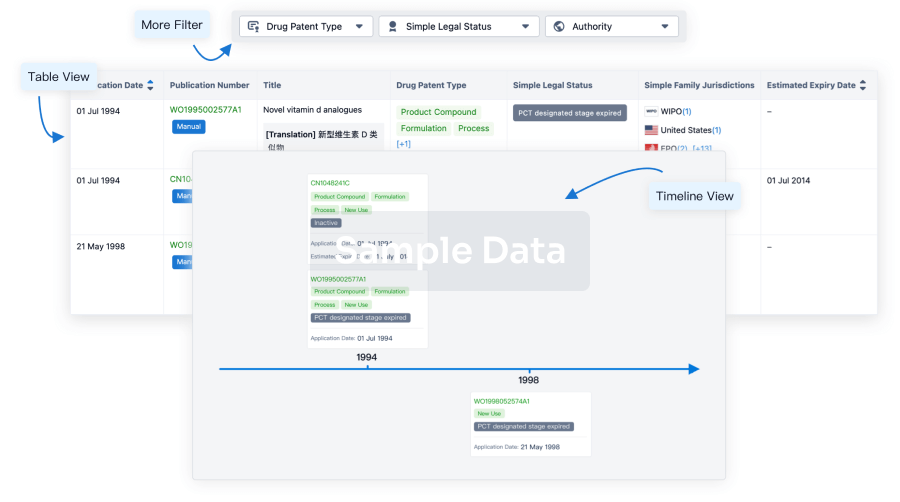
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
