Request Demo
Last update 17 May 2025
Dexelvucitabine
Last update 17 May 2025
Overview
Basic Info
Drug Type Small molecule drug |
Synonyms Dexelvucitabine (USAN/INN), DFC, RVT + [7] |
Target |
Action inhibitors |
Mechanism RT inhibitors(Viral reverse transcriptase inhibitors) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseDiscontinuedPhase 2 |
First Approval Date- |
Regulation- |
Login to view timeline
Structure/Sequence
Molecular FormulaC9H10FN3O3 |
InChIKeyHSBKFSPNDWWPSL-CAHLUQPWSA-N |
CAS Registry134379-77-4 |
Related
6
Clinical Trials associated with DexelvucitabineNCT03938545
ASCEND GO-2: A Phase 2b, Multicenter, Randomized, Double-blind, Placebo-controlled Study of RVT-1401 for the Treatment of Patients With Active, Moderate to Severe Graves' Ophthalmopathy
The purpose of the current study is to assess the efficacy and safety/tolerability of three dose regimens of RVT-1401 in the treatment of active, moderate to severe GO participants. In addition, the study is designed to characterize the effect of RVT-1401 exposure on reduction in anti-TSHR IgG
Start Date23 Jul 2019 |
Sponsor / Collaborator |
NCT03415282
Open-Label Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of RVT-501 Topical Ointment in Pediatric Patients With Atopic Dermatitis
This is a multicenter, open-label Phase 1b study in pediatric patients age 2-11 years old with extensive atopic dermatitis.
Start Date05 Feb 2018 |
Sponsor / Collaborator |
NCT03394677
Phase 2 Study to Evaluate the Efficacy, Safety, and Tolerabililty of RVT-501 Topical Ointment in Pediatric Patients With Mild to Moderate Atopic Dermatitis
This is a multi-center, randomized, vehicle controlled, double-blind Phase 2 study in pediatric patients age 2-17 years old with mild to moderate atopic dermatitis.
Start Date25 Jan 2018 |
Sponsor / Collaborator |
100 Clinical Results associated with Dexelvucitabine
Login to view more data
100 Translational Medicine associated with Dexelvucitabine
Login to view more data
100 Patents (Medical) associated with Dexelvucitabine
Login to view more data
349
Literatures (Medical) associated with Dexelvucitabine06 May 2025·Translational Vision Science & Technology
Distance From the Foveal Center: A Method for the Calculation of Eccentric Fixation
Article
Author: de Guimaraes, Thales A. C. ; Kalitzeos, Angelos ; Bainbridge, James ; Michaelides, Michel
Purpose:
The purpose of this study was to describe a method to determine the position of the preferred retinal locus (PRL).
Methods:
Cross-sectional data were obtained prospectively. Microperimetry was completed with the Macular Integrity Assessment (MAIA) under mesopic testing conditions. Spectral domain optical coherence tomography (SD-OCT) was acquired with the Spectralis SD-OCT system. The printout of the MAIA containing the PRL and both the horizontal and vertical transfoveal scans were interpolated in Adobe Photoshop, which was used to calculate the foveal center (FC) and to create a custom ruler to measure the distance from the foveal center (DFC) and the distance between PRL (DPRL). The methodology was tested by two independent graders in participants of a natural history study for KCNV2-associated retinopathy.
Results:
Twenty-two eyes of 12 subjects were analyzed at a mean age of 31.9 years (range = 11-54 years, SD = ±14.3). The mean DFC and DPRL was 1398 µm (range = 182.8-2896, SD = ±755.4), and 751.4 µm (range = 144.5-1493.3, SD = ±458.3) in the right eyes, and 1104 µm (range = 341.8-2513, SD = ±653.78) and 742.5 µm (range = 120-1918, SD = ±586.5) in the left eyes, respectively. There was no significant interocular correlation of DFC (r = -0.036, P = 0.92) or DPRL (r = 0.41, P = 0.26), or between the two variables in the right (r = 0.519, P = 0.084) and left eyes (r = 0.014, P = 0.97). These suggest that DPRL may not be related to the location of eccentric fixation and that the values of either eye are independent of each other.
Conclusions:
Our data suggest that these are reliable parameters, which could be of relevance in the context of clinical trials. The sample size is small and its correlation with other functional and structural outcomes remains to be explored, but these findings provide a framework for further development.
Translational Relevance:
This work bridges the distance between basic science and clinical care by providing a reliable and replicable method to quantify the preferred retinal locus of patients with eccentric fixation.
01 May 2025·Biomolecules & Therapeutics
Hepatoprotective Effects of Resveratrol on Acetaminophen-Induced Acute Liver Injury and Its Implications for Tofacitinib Disposition in Rats
Article
Author: Kim, So Hee ; Chang, Sun-Young ; Park, So Yeon ; Bae, Sung Hun ; Choi, Hyeon Gyeom
Tofacitinib, which is used to treat rheumatoid arthritis (RA), is primarily metabolized by the hepatic cytochrome P450 (CYP) enzymes, CYP3A1/2 and CYP2C11. Acetaminophen (APAP), which is frequently used for pain relief in patients with RA, can induce acute liver injury (ALI) when taken in excess, profoundly affecting drug metabolism. Resveratrol (RVT) is a polyphenolic compound with hepatoprotective properties. This study investigated the protective effects of RVT against APAP-induced ALI in rats, and explored its influence on the pharmacokinetics of tofacitinib. In ALI rats, both intravenous and oral administration of tofacitinib resulted in a significant (207% and 181%) increase in the area under the plasma concentration-time curve (AUC), primarily driven by a substantial reduction (66.1%) in non-renal clearance (CLNR) compared to that in control (CON) rats. Notably, RVT administration in ALI rats provided effective liver protection, partially restoring liver function, as evidenced by normalized glutamate oxaloacetate transaminase levels and the pharmacokinetic parameters, AUC and CLNR, closer to those observed in untreated CON rats (117% and 81.9%, respectively). These findings highlight the importance of considering the potential interactions between RVT or polyphenol-rich natural products and medications in patients with ALI in clinical practice.
01 May 2025·POULTRY SCIENCE
Growth patterns and heat tolerance analysis of dwarf chicken with frizzled feather
Article
Author: Li, Guo ; Bin, Chengfeng ; Wang, Xiaotong ; Ali, Sadaqat ; Zhang, Li ; Li, Lei ; Zhu, Zijing ; Xie, Tingting ; Guo, Dongxue ; Huang, Xunhe ; Zhang, Bing
Chickens are covered with feathers, lack sweat glands, and are sensitive to the thermal environment. Previously, our group bred a novel dwarf chicken strain with frizzled feather, named as dwarf chicken with frizzled feather (DFC). The cumulative growth of the chicken body weight and size were analyzed with 3 mathematical models. Subsequently, chickens were grouped to investigate the impact of heat stress (HS) on their slaughter performance, histomorphological development and gene mRNA change (HSP70, muscle development and appetite-related factors) using quantitative real-time PCR, tissue sections and Western Blot. In the HS group, chickens were placed at 34 ± 1°C for 8 hours (9:00 am - 17:00 pm) a day and lasted for 2 weeks, while in the control group, chickens were fed at 26 ± 1°C. Chicken tissue samples were collected at the age of 120 days to evaluate production performance, histological changes, and gene expression changes. Our results found that the Gompertz model was the best for fitting the body weight of DFC. The integrity of muscle, liver, spleen, and small intestine tissues was affected under HS conditions. Correspondingly, the length of the ileum was significantly decreased (P < 0.05), the thigh muscle development factor MYOD1 expression was down-regulated (P < 0.05), while the expression of MSTN was up-regulated (P < 0.001). In addition, the jejunum VH / CD was reduced significantly (P < 0.05). The mRNA of appetite-promoting factors AMPKα-1 and AGRP in the gut-brain axis were down-regulated (P < 0.05), while appetite-restrain factors CCK, GHRL, and CART were significantly up-regulated (P < 0.05 or P < 0.01). Moreover, the intestinal transport and absorption factors ZO1, OCLN, PepT1, SGLT1, and CAT1 were up-regulated (P < 0.05 or P < 0.01), and GLUT1 was down-regulated (P < 0.05). These results indicated that HS mainly impacted the appetite of chickens and did not significantly disrupt the nutrient absorption function of these chickens. The DFC appeared to be more tolerant to the hot environments for their frizzled feathers, small body size, and low basal metabolic rate.
2
News (Medical) associated with Dexelvucitabine09 Dec 2024
Dec. 04, 2024 -- Cidara Therapeutics, Inc. (Nasdaq: CDTX), a biotechnology company using its proprietary Cloudbreak® platform to develop drug-Fc conjugate (DFC) immunotherapies designed to save lives and improve the standard of care for patients facing serious diseases, today announced it has reached full planned enrollment of 5,000 subjects in the Phase 2b NAVIGATE trial across clinical sites in the US and UK. The trial is designed to evaluate the efficacy and safety of CD388, the company’s DFC for the pre-exposure prophylaxis of seasonal influenza.
“Completing the NAVIGATE study at the beginning of the northern hemisphere flu season was a critical milestone to evaluate the efficacy and safety of CD388 as a potential long-acting, universal influenza preventative,” said Jeffrey Stein, Ph.D., president and chief executive officer of Cidara. “Thanks to the efforts of our investigators and clinical teams, we are now well-positioned to advance the study as this year’s flu season unfolds.”
The Phase 2b NAVIGATE clinical trial is a randomized, double-blind, controlled trial in healthy, unvaccinated adult subjects who are not at risk of complications from influenza. The objective of the study is to evaluate safety, pharmacokinetics and the rates of laboratory and clinically confirmed influenza in subjects receiving the single doses of CD388 (150mg, 300mg, 450mg) or placebo administered once at the beginning of the flu season. Subjects are then followed for the remainder of the influenza season to monitor for breakthrough cases.
CD388 is an investigational drug-Fc conjugate (DFC) comprised of multiple copies of a potent small molecule neuraminidase inhibitor stably conjugated to a proprietary Fc fragment of a human antibody. DFCs are not vaccines or monoclonal antibodies but are low molecular weight biologics which are designed to function as long-acting small molecule inhibitors. CD388 was designed to provide universal protection against all known strains of seasonal and pandemic influenza with the potential to provide season-long protection with a single subcutaneous or intramuscular administration. Importantly, because CD388 is not a vaccine, its activity is not reliant on an immune response and thereby is expected to be efficacious in individuals regardless of immune status. More information can be found at: https://www.cidara.com/cloudbreak/influenza/.
Cidara Therapeutics is using its proprietary Cloudbreak® platform to develop novel drug-Fc conjugates (DFCs) comprising targeted small molecules or peptides coupled to a proprietary human antibody fragment (Fc). Cidara’s lead DFC candidate, CD388, is a long-acting antiviral designed to achieve universal prevention of seasonal and pandemic influenza with a single dose by directly inhibiting viral proliferation. In June 2023, CD388 was granted Fast Track Designation by the U.S. Food and Drug Administration (FDA), and the Company announced initiation of a Phase 2b trial in September 2024. Additional DFCs have been developed for oncology and in July 2024 Cidara received IND clearance for CBO421 which is intended to target CD73 in solid tumors. Cidara is headquartered in San Diego, California. For more information, please visit www.cidara.com.
The content above comes from the network. if any infringement, please contact us to modify.

ImmunotherapyFast TrackIND
29 Jun 2023
Data will be presented from an in vitro study of decellularized-flowable placental connective tissue matrix supporting cellular functions of human tenocytesFLORHAM PARK, N.J., June 29, 2023 (GLOBE NEWSWIRE) -- Celularity Inc. (Nasdaq: CELU) (Celularity), a biotechnology company developing placental-derived allogeneic cell therapies and biomaterial products, announced today that the Company will present data from a new product technology in development for orthopedic applications at the upcoming 7th International Conference on Orthopedics, July 19th – 20th in Rome. These data, which will be presented in an oral session entitled, “A Decellularized Flowable Placental Connective Tissue Matrix Supports Cellular Functions of Human Tenocytes In Vitro,” summarize Celularity’s recent benchtop studies indicating the potential therapeutic application of decellularized flowable placental connective tissue matrix (DF-CTM) for tendon repair. Tendon injuries are associated with considerable pain and disability. Tendon healing is controlled by tenocytes, a main tendon cell type, and their surrounding extracellular matrix, and is orchestrated through three multifaceted, overlapping stages, namely inflammatory, proliferative, and remodeling. Due to the hypovascularity and hypocellularity of the tendon tissue, natural tendon healing is slow and ineffective, and traditional conservative and surgical treatment options fail to address the underlying pathology. As a result, the healed tendon often is mechanically incompetent and prone to degeneration and rupture. Therefore, new methods are required to facilitate tendon repair and regeneration. Celularity’s hypothesis is that DF-CTM could provide structural and biochemical matrix components for tenocyte attachment, proliferation, and phenotype maintenance while decreasing inflammatory response. Celularity’s in vitro study tested tenocyte adhesion, proliferation, migration, phenotype maintenance, and inflammatory response when cultured on Celularity’s decellularized flowable placental CTM. Tenocyte proliferation and migration were significantly higher for DF-CTM than the control cultures (p = 0.005). In tenocytes cultured on DF-CTM, gene expressions indicative of tenocyte phenotype maintenance significantly increased over time (p < 0.001), including expressions of Scleraxis (SCX), a marker for differentiated tendon cells, Tenascin-C (TNC), an important factor in collagen fibril organization, Collagen type I (COL1A1), and Collagen type III (COL3A1). In tenocytes cultured on DF-CTM, the effects of TNF-α, an inflammatory cytokine, were diminished as indicated by significantly reduced expression of proinflammatory cytokines CXCL8 (p = 0.024) and MMP1 (p < 0.001). Over time, tenocytes cultured on DF-CTM promoted the expression of antifibrotic growth factor TGFβ3. In this in vitro evaluation, DF-CTM interacted favorably with human tenocytes as evidenced by tenocyte proliferation, maintenance of tenocyte phenotype, and attenuated inflammatory response. “We are very encouraged by the performance of our advanced placental-derived biomaterials, and we believe these data enhance our understanding of the potential application of DF-CTM for tendon repair, which could address a significant unmet need for patients with tendon injuries,” said Robert J. Hariri, M.D., Ph.D., Celularity’s CEO, Chairman and Founder. “Celularity leverages a diversified portfolio of therapeutic technologies all derived from a single source material — the post-partum placenta. Our novel business model is to research and develop proprietary cellular and regenerative therapeutics including biomaterial products. Celularity is unique in having a bifurcated manufacturing capability where biomaterial products represent a complementary revenue-generating opportunity that support our cellular therapy and regenerative medicine programs. It is our vision that cellular and biomaterial technologies may one day represent a means to create combination therapeutic strategies that harness natural regenerative processes and create more robust and durable clinical benefits. These early tenocyte data are an important demonstration of our ongoing commitment and focus on innovation leveraging the placental platform we have pioneered.” About Celularity Celularity Inc. (Nasdaq: CELU) headquartered in Florham Park, N.J., is a biotechnology company leading the next evolution in cellular and regenerative medicine by developing allogeneic cryopreserved off-the-shelf placental-derived cell therapies, including therapeutic programs using mesenchymal-like adherent stromal cells (MLASCs), T-cells engineered with CAR (CAR T-cells), and genetically modified and unmodified natural killer (NK) cells. These therapeutic programs target indications in autoimmune, infectious and degenerative diseases, and cancer. In addition, Celularity develops, manufactures, and commercializes innovative biomaterial products also derived from the postpartum placenta. Celularity believes that by harnessing the placenta’s unique biology and ready availability, it can develop therapeutic solutions that address significant unmet global needs for effective, accessible, and affordable therapies. To learn more, visit celularity.com Forward-Looking Statements This press release includes “forward-looking statements” within the meaning of The Private Securities Litigation Reform Act of 1995, as well as within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical facts are “forward-looking statements,” including those relating to future events. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “can,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “forecast,” “intends,” “may,” “might,” “outlook,” “plan,” “possible,” “potential,” “predict,” “project,” “seek,” “should,” “strive,” “target,” “will,” “would” and the negative of terms like these or other comparable terminology, and other words or terms of similar meaning. The forward-looking statements in this press release include express or implied statements regarding the potential application of DF-CTM for tendon repair, and Celularity’s ability to advance its technology platform to develop therapeutic options for unmet patient needs, among others. Many factors could cause actual results to differ materially from those described in these forward-looking statements, including but not limited to: the inherent risks in biotechnological development, including with respect to the development of novel biomaterial products and cellular therapies, and the clinical trial and regulatory approval process; and risks associated with Celularity’s current liquidity, as well as developments relating to Celularity’s competitors and industry, along with those risk factors set forth under the caption “Risk Factors” in Celularity’s annual report on Form 10-K filed with the Securities and Exchange Commission (SEC) on March 31, 2023, and other filings with the SEC. These risks and uncertainties may be amplified by current economic situations, including inflation, supply chain issues and overall economic uncertainty. If any of these risks materialize or underlying assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that Celularity does not presently know, or that Celularity currently believes are immaterial, that could also cause actual results to differ from those contained in the forward-looking statements. In addition, these forward-looking statements reflect Celularity’s current expectations, plans, or forecasts of future events and views as of the date of this communication. Subsequent events and developments could cause assessments to change. Accordingly, forward-looking statements should not be relied upon as representing Celularity’s views as of any subsequent date, and Celularity undertakes no obligation to update forward-looking statements to reflect events or circumstances after the date hereof, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Celularity Contact:Paul Graves, Chief Communications OfficerCelularity Inc.paul.graves@celularity.com
Cell TherapyClinical Study
100 Deals associated with Dexelvucitabine
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| D06580 | Dexelvucitabine | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| HIV Infections | Phase 2 | United States | 08 Sep 2003 | |
| Hepatitis B | Discovery | United States | 01 Oct 1999 | |
| Hepatitis B | Discovery | United States | 01 Oct 1999 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 65 | Placebo (Administered via subcutaneous injection) | lvycvyhmhj = nihzvawwki wlasvmqfig (cmcqfvssac, ctvfxeqckx - jdqdyvamtl) View more | - | 22 Sep 2022 | ||
Not Applicable | - | - | xbvktppudb(hwkokihygx) = gipmjzfewh ikeheqckaa (yleiheavyg, 2.9) View more | - | 01 Jan 2004 | ||
Not Applicable | - | nhdfnjhqsf(vlzchxelbc) = fsnnjgfsrs kdyfrpuymy (hznaerjiwb, 0.2) View more | - | 01 Jan 2003 | |||
nhdfnjhqsf(vlzchxelbc) = nypuxjlcyu kdyfrpuymy (hznaerjiwb, 1.6) |
Login to view more data
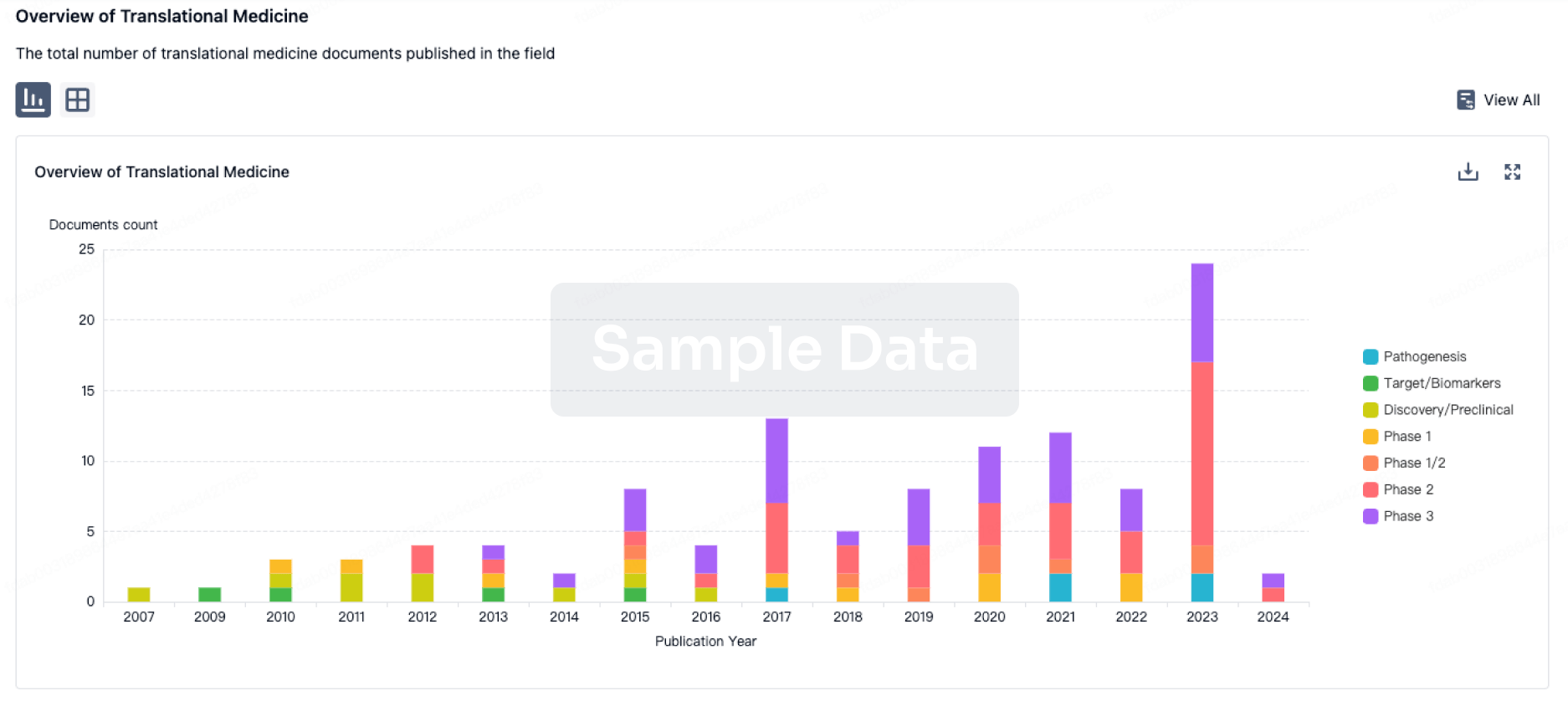
Translational Medicine
Boost your research with our translational medicine data.
login
or
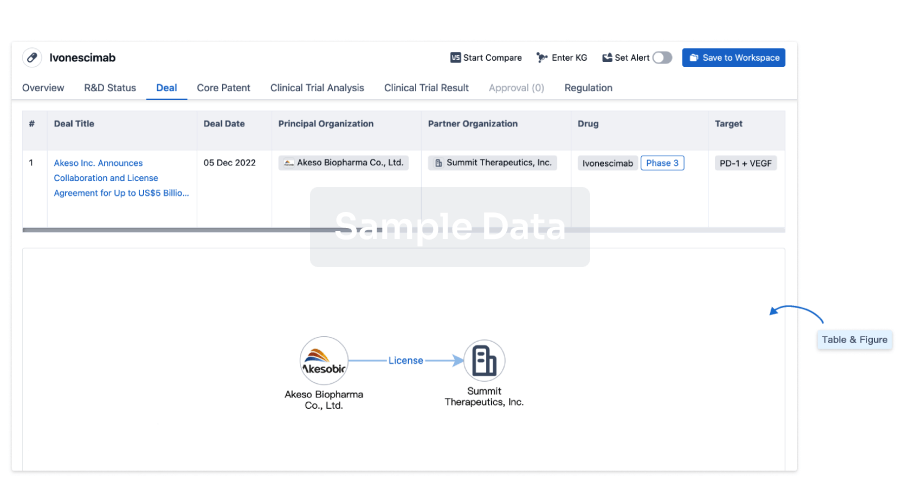
Deal
Boost your decision using our deal data.
login
or
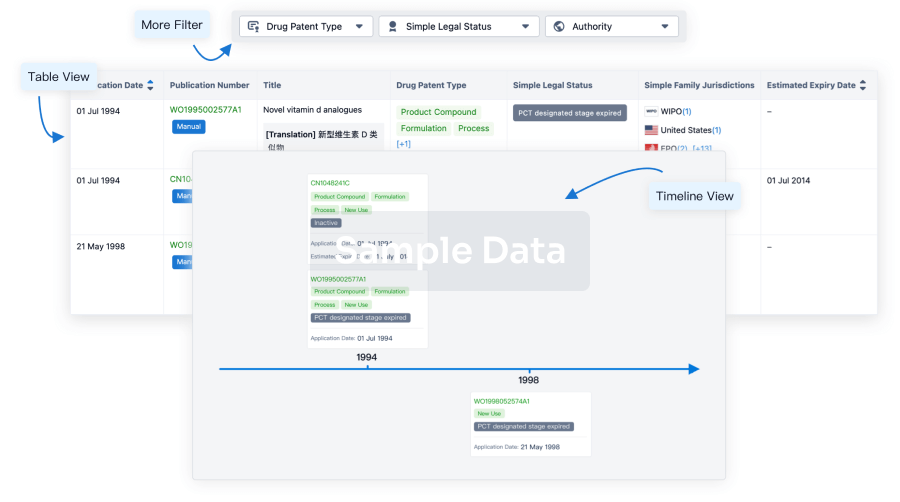
Core Patent
Boost your research with our Core Patent data.
login
or
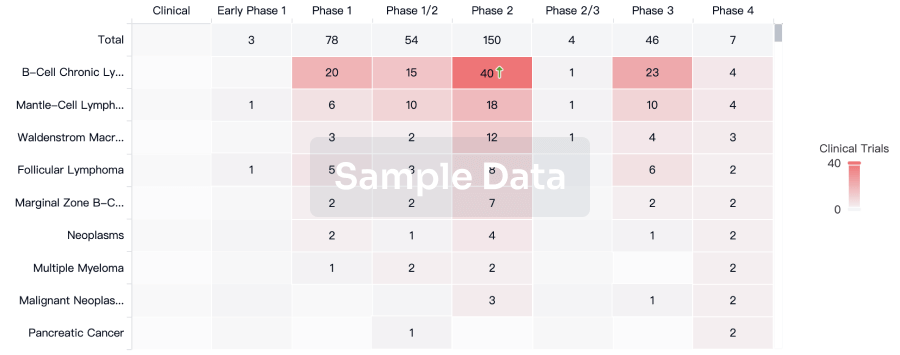
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or

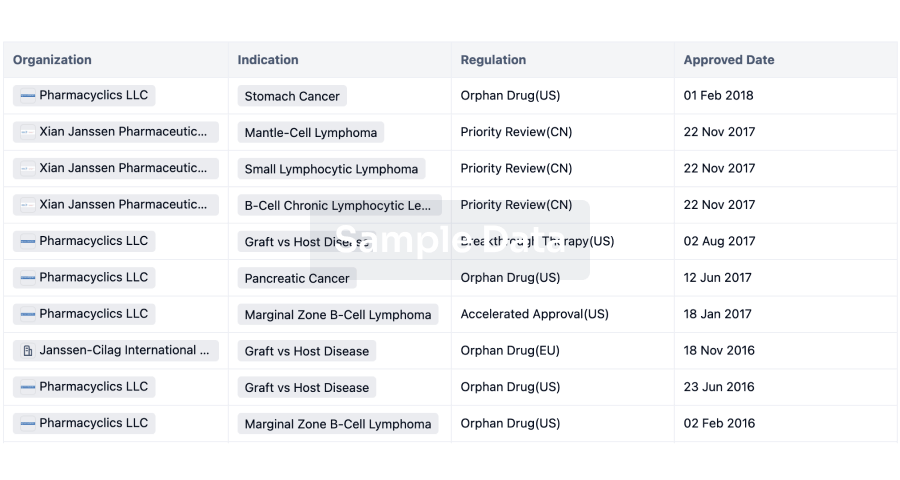
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free



