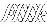Last update 29 Jun 2024
Tabalumab
Last update 29 Jun 2024
Overview
Basic Info
Drug Type Monoclonal antibody |
Synonyms Anti-BAFF MAb, Anti-BAFF monoclonal antibody, Tabalumab (genetical recombination) (JAN) + [3] |
Target |
Mechanism BAFF inhibitors(B-cell-activating factor/B lymphocyte stimulator inhibitors) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
Drug Highest PhaseDiscontinuedPhase 3 |
First Approval Date- |
RegulationOrphan Drug (US) |
Gene Sequence
Sequence Code 26440L

Source: *****
Sequence Code 192126H

Source: *****
Related
44
Clinical Trials associated with TabalumabPharmacokinetic Evaluations of Tabalumab Following Subcutaneous Administration by Prefilled Syringe or Auto Injector in Patients With Systemic Lupus Erythematosus
The purpose of this study is to evaluate the amount of tabalumab in the blood after it is given by two different injection methods - A traditional syringe or a spring loaded syringe for 12 weeks. Participants may continue to receive study drug for up to 52 weeks.
Start Date01 Jan 2014 |
Sponsor / Collaborator |
A Phase 3b, Multicenter, Open-Label Study to Evaluate the Long-Term Safety and Efficacy of Subcutaneous LY2127399 in Patients with Systemic Lupus Erythematosus (SLE)
Start Date10 Jun 2013 |
Sponsor / Collaborator |
A PHASE 3, MULTICENTER, RANDOMIZED, DOUBLED-BLIND, PLACEBO-CONTROLLED STUDY TO EVALUATE THE EFFICACY AND SAFETY OF LY2127399 IN PATIENTS WITH MODERATE TO SEVERE RHEUMATOID ARTHRITIS (RA) WHO HAD AN INADEQUATE RESPONSE TO ONE OR MORE TNF-α INHIBITORS
Start Date07 Jan 2013 |
Sponsor / Collaborator |
100 Clinical Results associated with Tabalumab
Login to view more data
100 Translational Medicine associated with Tabalumab
Login to view more data
100 Patents (Medical) associated with Tabalumab
Login to view more data
260
Literatures (Medical) associated with Tabalumab01 Oct 2023·iScience
Transchromosomic bovine-derived anti-SARS-CoV-2 polyclonal human antibodies protects hACE2 transgenic hamsters against multiple variants
Article
Author: Li, Rong ; Wang, Zhongde ; Lundy, Jeneveve ; Wu, Hua ; Bausch, Christoph ; Alcorn, Maria D H ; Klimstra, William B ; Vasilatos, Shauna ; Nambulli, Sham ; Sullivan, Eddie ; Egland, Kristi ; Duprex, Paul ; Terada, Yutaka ; Luke, Thomas ; Gilliland, Theron ; Liu, Yanan ; Dunn, Matthew ; Larson, Deanna
Pandemic SARS-CoV-2 has undergone rapid evolution resulting in the emergence of many variants with mutations in the spike protein, some of which appear to evade antibody neutralization, transmit more efficiently, and/or exhibit altered virulence. This raises significant concerns regarding the efficacy of anti-S monoclonal antibody-based therapeutics which have failed against variant SARS-CoV-2 viruses. To address this concern, SAB-185, a human anti-SARS-CoV-2 polyclonal antibody was generated in the DiversitAb platform. SAB-185 exhibited equivalent, robust in vitro neutralization for Munich, Alpha, Beta, Gamma, and Δ144-146 variants and, although diminished, retained PRNT50 and PRNT80 neutralization endpoints for Delta and Omicron variants. Human ACE2 transgenic Syrian hamsters, which exhibit lethal SARS-CoV-2 disease, were protected from mortality after challenge with the Munich, Alpha, Beta, Delta, and Δ144-146 variants and clinical signs after non-lethal Omicron BA.1 infection. This suggests that SAB-185 may be an effective immunotherapy even in the presence of ongoing viral mutation.
01 Sep 2023·Acta pharmacologica Sinica
Inflachromene attenuates seizure severity in mouse epilepsy models via inhibiting HMGB1 translocation.
Article
Author: Park, Seung Bum ; Yi, Sihyeong ; Wang, Yi ; Shao, Yu-Ying ; Shi, Jia-Ying ; Qiu, Xiao-Yun ; Yan, Meng-Qi ; Dai, Si-Jie ; Xu, Ceng-Lin ; Cho, Wan-Sang ; Sun, Jin-Yi ; Zheng, Yang ; Nishibori, Masahiro ; Chen, Zhong ; Li, Zhi-Sheng
Epilepsy is not well controlled by current anti-seizure drugs (ASDs). High mobility group box 1 (HMGB1) is a DNA-binding protein in the nucleus regulating transcriptional activity and maintaining chromatin structure and DNA repair. In epileptic brains, HMGB1 is released by activated glia and neurons, interacting with various receptors like Toll-like receptor 4 (TLR4) and downstream glutamatergic NMDA receptor, thus enhancing neural excitability. But there is a lack of small-molecule drugs targeting the HMGB1-related pathways. In this study we evaluated the therapeutic potential of inflachromene (ICM), an HMGB-targeting small-molecule inhibitor, in mouse epilepsy models. Pentylenetetrazol-, kainic acid- and kindling-induced epilepsy models were established in mice. The mice were pre-treated with ICM (3, 10 mg/kg, i.p.). We showed that ICM pretreatment significantly reduced the severity of epileptic seizures in all the three epilepsy models. ICM (10 mg/kg) exerted the most apparent anti-seizure effect in kainic acid-induced epileptic status (SE) model. By immunohistochemical analysis of brain sections from kainic acid-induced SE mice, we found that kainic acid greatly enhanced HMGB1 translocation in the hippocampus, which was attenuated by ICM pretreatment in subregion- and cell type-dependent manners. Notably, in CA1 region, the seizure focus, ICM pretreatment mainly inhibited HMGB1 translocation in microglia. Furthermore, the anti-seizure effect of ICM was related to HMGB1 targeting, as pre-injection of anti-HMGB1 monoclonal antibody (5 mg/kg, i.p.) blocked the seizure-suppressing effect of ICM in kainic acid-induced SE model. In addition, ICM pretreatment significantly alleviated pyramidal neuronal loss and granule cell dispersion in kainic acid-induced SE model. These results demonstrate that ICM is an HMGB-targeting small molecule with anti-seizure potential, which may help develop a potential drug for treating epilepsy.
01 Aug 2023·Pathology, research and practice
Manifestation of acute appendicitis as known but paradox visceral side effect of ulcerative colitis anti-inflammatory therapy with januskinase-inhibitor Tofacitinib (Xeljanz™)
Author: Meyer, F. ; Weber, F. ; Eger, K. I. ; March, C. ; Croner, R. S.
BACKGROUND:
The etiopathogenesis of accompanying inflammatory phenomena and consequences of immunomodulation constitute a challenging and innovative field in the medical treatment of patients with autoimmune diseases.
AIM:
Based on i) clinical management experience gained from this challenging clinical case and ii) selective references of reports published in the scientific medical literature, we present an unusual counterfactual scientific case report. A patient diagnosed with ulcerative colitis undergoing januskinase (JAK)-inhibitor therapy developed acuteappendicitis as an unusual complication or as a visceral side effect of immunosuppressive/anti-inflammatory therapy.
METHOD:
Scientific case report.
RESULTS:
(case description): Medical history: A 52-year-old male presented with spasmodic pain in the right lower abdomen lasting for two days (no fever, no bowel movement changes (no stool irregularities), no vomiting).
MEDICATION USED TO DATE:
Steroid-resistant ulcerative colitis treated with immunosuppressive therapy (Adalimumab administered for 10 months [next generation anti-TNFα mAb], Vendolizumab for 9 months [α4β7 integrin antagonist], Tofacitinib for 6 months); fructose intolerance, no previous abdominal surgery; medication: XeljanzTM (Tofacitinib, 5 mg 2x1; JAK-inhibitor; PFIZER PHARMA GmbH, Berlin,Germany); MutaflorTM (1x1; Ardeypharm GmbH, Herdecke, Germany).
CLINICAL FINDINGS:
Pressure pain in the right lower abdomen with local muscular defense (Mc-Burney's/Lanz's point positive), no peritonism, Psoas-muscle sign positive.
DIAGNOSTIC MEASURES:
Laboratory parameters: standard value of white blood cell count, CrP: 25 mg/l.-Transabdominal ultrasound revealed hypertrophic 'appendix vermiformis' with detectable target-phenomenon and surrounding fluid.
DECISION-MAKING:
Indication for laparoscopic exploration.
THERAPY:
Under perioperative single-shot antibiotic administration with UnacidTM, the patient underwent emergency laparoscopic appendectomy due to confirmed acute appendicitis with additional lavage and placement of local drainage.
CLINICAL COURSE:
The postoperative phase was uneventful (sufficient analgetic therapy, removal of local drainage on the 2nd postoperative day). The patient was discharged four days after surgery. Histopathology confirmed ulcero-phlegmonous, acute purulent appendicitis with fibrinous purulent mesenteriolitis.
FURTHER MEASURES:
Immunosuppressive therapy was continued.
CONCLUSION:
Based on the paradoxon of an acute inflammatory disease (acute appendicitis) seen in the case of a patient undergoing immunosuppressive/anti-inflammatory treatment using a JAK-Inhibitor for ulcerative colitis, we consider this case worthy of publication although this side effect has previously been described in patients with rheumatoid arthritis. This might be the manifestation of i) an immunomodulatory effect that reduced or at least altered mucosal defense, including an increased risk of opportunistic infections, presenting as a specific visceral 'side effect' of the JAK-Inhibitor and/or as a consequence; ii) an induced alternative inflammatory mechanism/proinflammatory signal transduction and - theoretically - an intestinal drainage defect in the segment of right colic artery with consecutive collection of necrotic cells and activation of inflammatory mediators.
20
News (Medical) associated with Tabalumab14 Jun 2024
Tibulizumab (ZB-106) was well tolerated and neutralized IL-17A and BAFF in a Phase 1 study in patients with Sjogren’s syndrome
Preclinical data demonstrating the potential of dual inhibition of IL-17A and BAFF in a rheumatoid arthritis animal model support clinical development
HENDERSON, Nev.--(BUSINESS WIRE)-- Zura Bio Limited (Nasdaq: ZURA) (“Zura Bio”), a clinical-stage immunology company developing novel dual-pathway antibodies for autoimmune and inflammatory diseases, today shared supportive data from a Phase 1 study evaluating its lead candidate, tibulizumab (ZB-106), for the treatment of Sjogren’s syndrome. These data, along with preclinical data supporting further development of tibulizumab in rheumatoid arthritis (RA), were presented at the Annual European Congress of Rheumatology (EULAR) 2024 in Vienna.
“Collectively, these data add to early-phase evidence demonstrating that dual-inhibition of both IL-17A and BAFF could be a breakthrough approach for autoimmune and inflammatory diseases in which single-pathway inhibition is the standard of care,” stated Robert Lisicki, Chief Executive Officer. “The results in Sjogren’s syndrome demonstrate that tibulizumab achieved robust target engagement, nearing maximum serum levels following single, well-tolerated subcutaneous doses at four-week intervals. Further, the preclinical results suggest dual-pathway inhibition may warrant clinical exploration in RA and other autoimmune diseases, adding to the breadth of potential we see with tibulizumab.”
Key Findings from Phase 1 Study of Tibulizumab in Sjogren’s Syndrome
The randomized, double-blind, placebo-controlled Phase 1 study evaluated four ascending doses of tibulizumab in 25 participants with Sjogren’s syndrome. Twenty-one participants in the 12-week study received ≥1 dose of tibulizumab (30mg Q4W, 100mg Q4W, 300mg Q4W, 300mg Q2W), with four receiving placebo.
Treatment with tibulizumab was generally well tolerated in patients with Sjogren’s syndrome.
Serum levels of total IL-17A and BAFF increased following tibulizumab administration, reflecting target engagement. At doses of 100 mg Q4W and higher, the total IL-17A and BAFF concentrations appeared to plateau, suggesting the targets were engaged nearly to maximum levels.
Throughout the study, total B cell counts were dose-dependently reduced in all participants, while administration of tibulizumab was associated with lower levels of Th1 cells. Tibulizumab was also shown to modulate inflammatory mediators, including serum amyloid A, interleukins 5 and 10, as well as basic fibroblast growth factor. These reductions suggest tibulizumab has treatment potential for additional autoimmune conditions.
The poster is available in the News and Events section on the Zura Bio website and will be archived for at least 30 days following presentation.
Key Findings from Preclinical Study of Tibulizumab in an RA Model
The preclinical study was designed to evaluate the respective and combined benefits of inhibiting IL-17A and BAFF in a mouse model of RA. Mice received IL-17A antibodies and/or BAFF antibodies, or a control antibody.
Cumulative clinical disease scores were significantly reduced in mice treated with the combination of anti-IL-17A and anti-BAFF compared to the isotype control (p<0.001); combined IL-17A and BAFF inhibition also resulted in less signs of disease compared to individually targeted treatment.
Combined IL-17A and BAFF inhibition reduced inflammation significantly compared to the control (p<0.05).
Combined IL-17A and BAFF inhibition was associated with a significant reduction of anti-collagen antibodies compared to the control (p<0.01).
The study abstract, which was accepted for publication only, is available on the EULAR website.
ABOUT TIBULIZUMAB
Tibulizumab, a humanized bispecific dual antagonist antibody, is a fusion of TALTZ® (ixekizumab) and tabalumab that has been engineered to bind to and neutralize both IL-17A and BAFF. Tibulizumab is expected to enter Phase 2 clinical development for the treatment of systemic sclerosis in Q4-2024 and hidradenitis suppurativa in Q2-2025. Completed tibulizumab studies include Phase 1/1b trials in Sjogren’s syndrome and rheumatoid arthritis.
ABOUT ZURA BIO
Zura Bio is a clinical-stage, multi-asset immunology company developing novel dual-pathway antibodies for autoimmune and inflammatory diseases. Currently, Zura Bio is developing three assets which have completed Phase 1/1b studies and are Phase 2 ready. The company is developing a portfolio of therapeutic indications for tibulizumab (ZB-106), ZB-168, and torudokimab (ZB-880), with a goal of demonstrating their efficacy, safety, and dosing convenience in autoimmune and inflammatory diseases, including systemic sclerosis and other novel indications with unmet needs.
FORWARD-LOOKING STATEMENTS
This communication includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believe,” “predict,” “potential,” “continue,” “strategy,” “future,” “opportunity,” “would,” “seem,” “seek,” “outlook” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties that could cause the actual results to differ materially from the expected results. These statements are based on various assumptions, whether or not identified in this communication. These forward-looking statements in this release include, but are not limited to, statements regarding Zura Bio’s anticipated proceeds to be received in the proposed Private Placement, expected timing of closing of the proposed Private Placement and the size, completion and use of proceeds of the proposed Private Placement, the forecast of cash runway and the Company’s expectations regarding funding, operating and working capital expenditures, business strategies and objectives, statements related to Zura Bio’s abilities to achieve anticipated internal readouts and achieve them in expected time periods, Zura Bio’s product candidates, clinical trials and the design and timing thereof, statements with respect to expected therapeutic potential and statements regarding Zura Bio’s product candidates ability to proceed into Phase 2 clinical trials. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability.
Actual events and the ability to consummate the proposed Private Placement and the timing and proceeds thereof; are difficult or impossible to predict and could differ materially from those expressed or implied in such forward-looking statements. You should carefully consider the risks and uncertainties described in the “Risk Factors” sections of Zura Bio's 10-K for the year ended December 31, 2023 and other filings with the SEC, including: Zura Bio’s expectations regarding product candidates and their related benefits; Zura Bio’s beliefs regarding potential benefits or limitations of competing products both in development and approved; information regarding Zura Bio’s vision and strategy; anticipated timing of key events and initiation of Zura Bio’s studies and release of clinical data; Zura Bio’s expectations regarding the general acceptability and maintenance of our products by regulatory authorities, payors, physicians, and patients; Zura Bio’s ability to attract and retain key personnel; the accuracy of Zura Bio’s future operating expenses, capital requirements and needs for additional financing; Zura Bio’s ability to obtain funding for operations, including funds that may be necessary to complete development of our product candidates; the fact that Zura Bio has not completed any clinical trials and has no products approved for commercial sale; the fact that Zura Bio has incurred significant losses since inception, and it expects to incur significant losses for the foreseeable future and may not be able to achieve or sustain profitability in the future; Zura Bio’s ability to renew existing contracts; Zura Bio’s reliance on third-party contract development manufacturing organizations for the manufacture of clinical materials; Zura Bio’s ability to obtain regulatory approval for our products, and any related restrictions or limitations of any approved products; Zura Bio’s ability to effectively manage growth and competitive pressures from other companies worldwide in the therapies in which Zura Bio competes; and litigation and Zura Bio’s ability to adequately protect intellectual property rights. These risks and uncertainties may be amplified by health epidemics or other unanticipated global disruption events, which may continue to cause economic uncertainty. Zura Bio cautions that the foregoing list of factors is not exclusive or exhaustive and not to place undue reliance upon any forward-looking statements, including projections, which speak only as of the date made. Zura Bio gives no assurance that it will achieve its expectations. Zura Bio does not undertake or accept any obligation to publicly provide revisions or updates to any forward-looking statements, whether as a result of new information, future developments or otherwise, or should circumstances change, except as otherwise required by securities and other applicable laws.
View source version on businesswire.com:
Contacts
Megan K. Weinshank
Head of Investor Relations
ir@zurabio.com
Source: Zura Bio Limited
View this news release online at:
Clinical ResultPhase 1Phase 2
28 Mar 2024
Significant 2023 milestones highlighted by successful Nasdaq listing, leadership team buildout, formation of strategic development and scientific plan
On track to initiate Phase 2 study evaluating tibulizumab (ZB-106) for the treatment of systemic sclerosis (SSc) in 2H 2024
Appointment of CEO, Robert Lisicki, underscores Zura Bio’s commitment to building leading immunology company as we prioritize excellence in strategic development and scientific planning
Outgoing Founder and CEO, Someit Sidhu, M.D., will continue as a Board Director to leverage institutional knowledge and provide continued support
Cash position as of December 31, 2023 totaling $99.8 million in cash, cash equivalents, and investments expected to support development and operations into 2026
HENDERSON, Nev--(BUSINESS WIRE)-- Zura Bio Limited (Nasdaq: ZURA) (“Zura Bio”) a clinical-stage immunology company developing novel dual-pathway antibodies for autoimmune and inflammatory diseases, today reported full year 2023 financial results and recent business highlights. The Company has also announced the appointment of Robert Lisicki as Chief Executive Officer (CEO), effective April 8, 2024, succeeding its Founding CEO, Someit Sidhu, M.D. Dr. Sidhu will continue to serve Zura Bio as a non-independent Board Director.
“Throughout 2023, Zura Bio achieved significant milestones by successfully listing on Nasdaq, broadening our portfolio of differentiated clinical-stage immunology and inflammation assets, and building our management team. These accomplishments have firmly established a robust operational framework for our business that is underpinned by a cash runway expected to fund operations into 2026,” stated Someit Sidhu, M.D., Founder and CEO of Zura Bio, “We remain focused on initiating a Phase 2 clinical trial evaluating our lead asset, tibulizumab, in systemic sclerosis in the second half of 2024. This involves effective management of our development and manufacturing partners, the thoughtful selection of a contract research organization, and communications with regulatory authorities. Concurrently, we're advancing ZB-168 and torudokimab towards Phase 2 readiness while closely monitoring external readouts expected in 2024.”
Zura Bio has appointed Robert Lisicki as CEO, effective April 8, 2024, succeeding Dr. Sidhu, who remains actively involved in the Company as a non-independent Director of the Board. The Company will expand its Board membership to ten, which includes Mr. Lisicki’s role as a Director of the Board. Mr. Lisicki joined Zura Bio in January 2024 as President and Chief Operating Officer.
Dr. Sidhu continued, "On behalf of the Management Team and Board, I am pleased to welcome Robert as the Chief Executive Officer. Robert brings a wealth of industry leadership experience complemented by expertise in drug development and commercial operations. With his proven track record of excellence, he is the right individual to lead Zura Bio as our lead asset enters Phase 2 development. I will work closely with Robert to transition CEO responsibilities and remain actively involved as a Director."
IMPORTANT UPCOMING ANTICIPATED EVENTS FOR ZURA BIO
Tibulizumab (ZB-106): Zura Bio plans to initiate a Phase 2 trial of tibulizumab for the treatment of systemic sclerosis in 2H 2024. Tibulizumab is a tetravalent dual-antagonist antibody engineered by the fusion of TALTZ® (ixekizumab) and tabalumab that neutralizes IL-17A and BAFF.
ZB-168: Zura Bio plans to conduct necessary CMC and regulatory activities to prepare ZB-168, an anti-IL-7Rα inhibitor, for Phase 2 readiness. Additionally, the Company is actively monitoring Phase 2 IL-7R external catalysts in ulcerative colitis, atopic dermatitis, and alopecia areata, along with additional TSLP-driven catalysts.
Torudokimab (ZB-880): Zura Bio plans to conduct necessary CMC and regulatory activities to prepare torudokimab, an anti-IL-33 antibody, for Phase 2 readiness in allergy or respiratory-related indications. Additionally, the Company is monitoring Phase 2 and Phase 3 external catalysts in asthma and chronic obstructive pulmonary disease.
BUSINESS AND FINANCIAL HIGHLIGHTS
Raised approximately $145 million in 1H 2023 to support our pipeline and business operations.
In March 2023, successfully closed a Business Combination Agreement (BCA) with JATT Acquisition Corp., resulting in approximately $65 million in gross cash proceeds. Simultaneously, began trading on Nasdaq under the ticker symbol ZURA.
In April 2023, completed $80 million financing from top institutional biotech investors supporting the in-licensing of tibulizumab, a potential first-in-class anti-IL-17 and anti-BAFF tetravalent bispecific antibody, from Eli Lilly and Company.
Enhanced and expanded the Board of Directors and Executive leadership team to fortify business operations and ensure readiness for clinical trials in 2024.
In April 2023, appointed Michael Howell, Ph.D. as the Chief Scientific Officer and Head of Translational Medicine.
In November 2023, appointed Arnout Ploos van Amstel to the Board of Directors.
In January 2024, appointed Kiran Nistala, M.B.B.S., Ph.D. as the Chief Medical Officer and Head of Development.
In March 2024, appointed Robert Lisicki to CEO, effective April 8, 2024.
Joined the Russell 2000® and Russell 3000® Indexes in June 2023.
Entered into a sponsored research agreement with Benaroya Research Institute in September 2023 to further characterize the pivotal role of Interleukin-7 receptor alpha (IL-7Rα) in Thymic Stromal Lymphopoietin (TSLP) and Interleukin-7 (IL-7) signaling pathways.
Presented results from two abstracts at the World Allergy Congress in December 2023, highlighting the Company’s informative research for ZB-168 and torudokimab.
Cash and cash equivalents: Cash and cash equivalents were $99.8 million as of December 31, 2023, as compared to $1.6 million as of December 31, 2022. The increased cash balance is primarily due to the aggregate of capital raised from closing of the BCA in March 2023 and the private placement transaction in April 2023. Zura Bio anticipates that its cash and cash equivalents are sufficient to fund planned operations into 2026.
Research and Development (R&D) expenses: R&D expenses were $44.0 million for the year ended December 31, 2023, an increase of $20.3 million compared to $23.7 million for the year ended December 31, 2022. The increase was primarily due to an increase of $9.1 million related to manufacturing, and an increase of $7.5 million of costs incurred to acquire licenses. Additionally, there was an increase of $1.5 million in expenses related to compensation for personnel, including share-based compensation.
General and Administrative (G&A) expenses: G&A expenses were $18.6 million for the year ended December 31, 2023, an increase of $15.2 million compared to the $3.5 million for the year ended December 31, 2022. The increase was primarily due to an increase of $11.4 million in compensation for personnel in G&A functions, including share-based compensation as well as an increase of $2.9 million in professional services.
Net loss: Net loss for the year ended December 31, 2023 was $60.4 million compared to $25.7 million for the year ended December 31, 2022.
ABOUT ZURA BIO
Zura Bio is a clinical-stage, multi-asset immunology company developing novel dual-pathway antibodies for autoimmune and inflammatory diseases. Currently, Zura Bio is developing three assets which have completed Phase 1/1b studies and are Phase 2 ready. The company is developing a portfolio of therapeutic indications for tibulizumab, ZB-168, and torudokimab with a goal of demonstrating their efficacy, safety, and dosing convenience in autoimmune and inflammatory diseases, including systemic sclerosis and other novel indications with unmet needs.
FORWARD-LOOKING STATEMENTS
This communication includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believe,” “predict,” “potential,” “continue,” “strategy,” “future,” “opportunity,” “would,” “seem,” “seek,” “outlook” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties that could cause the actual results to differ materially from the expected results. These statements are based on various assumptions, whether or not identified in this communication. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability.
Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. You should carefully consider the risks and uncertainties described in the “Risk Factors” sections of Zura Bio’s recent filings with the SEC. These filings would identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Many of these factors are outside Zura Bio’s control and are difficult to predict. Many factors could cause actual future events to differ from the forward-looking statements in this communication, including but not limited to: (1) the outcome of any legal proceedings that may be instituted against Zura Bio; (2) volatility in the price of Zura Bio’s securities; (3) the ability of Zura Bio to successfully conduct research and development activities, grow and manage growth profitably, maintain relationships with customers and suppliers, and retain key employees; (4) the ongoing costs relating to operating as a public company; (5) changes in the applicable laws or regulations; (6) the possibility that Zura Bio may be adversely affected by other economic, business, and/or competitive factors; (7) the risk of downturns and a changing regulatory landscape in the highly competitive industry in which Zura Bio operates; (8) the potential inability of Zura Bio to raise additional capital needed to pursue its business objectives or to achieve efficiencies regarding other costs; (9) the enforceability of Zura Bio’s intellectual property, including its patents, and the potential infringement on the intellectual property rights of others, cyber security risks or potential breaches of data security; and (10) other risks and uncertainties described in the registration statement on Form S-1 filed with the SEC on June 14, 2023, and such other documents filed by Zura Bio from time to time with the SEC. These risks and uncertainties may be amplified by the COVID-19 pandemic or other unanticipated global disruption events, which may continue to cause economic uncertainty. Zura Bio cautions that the foregoing list of factors is not exclusive or exhaustive and not to place undue reliance upon any forward-looking statements, including projections, which speak only as of the date made. Zura Bio gives no assurance that it will achieve its expectations. Zura Bio does not undertake or accept any obligation to publicly provide revisions or updates to any forward-looking statements, whether as a result of new information, future developments or otherwise, or should circumstances change, except as otherwise required by securities and other applicable laws.
ZURA BIO LIMITED
CONSOLIDATED BALANCE SHEETS
December 31,
December 31,
2023
2022
Assets
Current assets:
Cash and cash equivalents
$
99,806
$
1,567
Prepaid expenses and other current assets
1,037
209
Total current assets
100,843
1,776
Deferred offering costs
—
3,486
Total assets
$
100,843
$
5,262
Liabilities, Redeemable Noncontrolling Interest, Convertible Preferred Shares, and Shareholders’ Equity (Deficit)
Current liabilities:
Accounts payable and accrued expenses
$
20,302
$
4,428
Note payable
—
7,756
Research and development license consideration liability
—
2,634
Total current liabilities
20,302
14,818
Private placement warrants
990
—
Total liabilities
21,292
14,818
Commitments and contingencies (Note 12)
Redeemable noncontrolling interest
18,680
10,000
Convertible preferred shares
Series A-1 convertible preferred shares, $0.001 par value, -0- and 13,510,415 shares authorized, issued and outstanding as of December 31, 2023 and 2022, respectively
—
12,500
Shareholders’ Equity (Deficit):
Preferred Shares, $0.0001 par value, 1,000,000 and -0- authorized as of December 31, 2023 and 2022, respectively; -0- issued and outstanding as of December 31, 2023 and 2022
—
—
Class A Ordinary Shares, $0.0001 par value, 300,000,000 authorized, 43,593,678 issued and outstanding as of December 31, 2023; 1,884,649 authorized, 279,720 issued and outstanding as of December 31, 2022
4
—
Additional paid-in capital
162,820
—
Accumulated deficit
(103,494
)
(32,056
)
Total Zura Bio Limited shareholders’ equity (deficit)
59,330
(32,056
)
Noncontrolling interest
1,541
—
Total shareholders’ equity (deficit)
60,871
(32,056
)
Total liabilities, redeemable noncontrolling interest, convertible preferred shares, and shareholders’ equity (deficit)
$
100,843
$
5,262
ZURA BIO LIMITED
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except share and per share data)
For the Period from
January 18, 2022
For the Year Ended
(date of inception) to
December 31,
December 31,
2023
2022
Operating expenses:
Research and development
$
43,999
$
23,689
General and administrative
18,639
3,473
Total operating expenses
62,638
27,162
Loss from operations
(62,638
)
(27,162
)
Other expense/(income), net:
Other expense, net
(17
)
23
Interest income
(2,186
)
(8
)
Dividend income
(1,392
)
—
Change in fair value of private placement warrants
(724
)
—
Change in fair value of note payable
2,244
156
Total other expense/(income), net
(2,075
)
171
Loss before income taxes
(60,563
)
(27,333
)
Income tax benefit
—
—
Net loss before redeemable noncontrolling interest
(60,563
)
(27,333
)
Net loss attributable to redeemable noncontrolling interest
203
1,595
Net loss
(60,360
)
(25,738
)
Accretion of redeemable noncontrolling interest to redemption value
(7,220
)
(6,652
)
Deemed contribution from redeemable noncontrolling interest
9,212
—
Deemed dividend to redeemable noncontrolling interest
(10,875
)
—
Net loss attributable to Class A Ordinary Shareholders of Zura
$
(69,243
)
$
(32,390
)
Net loss per share attributable to Class A Ordinary Shareholders of Zura, basic and diluted
$
(2.09
)
$
(141.97
)
Weighted-average Class A Ordinary Shares used in computing net loss per share attributable to Class A Ordinary Shareholders of Zura, basic and diluted
33,064,036
228,148
View source version on businesswire.com:
Contacts
Megan K. Weinshank
Head of Investor Relations
IR@zurabio.com
Lee M. Stern
Meru Advisors
lstern@meruadvisors.com
Source: Zura Bio Limited
View this news release online at:
Executive ChangePhase 2Financial StatementPhase 1
04 Dec 2023
HENDERSON, Nev.--(
BUSINESS WIRE
)--
Zura Bio Limited
(Nasdaq: ZURA) (“Zura Bio”), a multi-asset clinical-stage biotechnology company focused on developing novel medicines for immune and inflammatory disorders, today announced two abstracts were accepted and presented at
World Allergy Congress
(WAC), held December 1 – 3, 2023 in Bangkok, Thailand. The abstracts highlight Zura Bio’s informative research for ZB-168, an anti-IL-7Rα monoclonal antibody with potential across a broad set of indications where the IL-7 or TSLP pathways may be involved, and ZB-880 (torudokimab), a fully human, high affinity monoclonal antibody that neutralizes IL-33 and also has potential for a broad range of indications.
“
Introducing Zura Bio’s ZB-168 and ZB-880 at World Allergy Congress is an opportunity to share the unique science behind these assets. ZB-168 uniquely inhibits both IL-7 and TSLP, while ZB-880 prevents IL-33 mediated-activation of ST2-dependent and independent inflammation. The unique biology of our assets may give us access to a broad range of biology applicable in a number of autoimmune and inflammatory diseases,” said Michael Howell, Ph.D., Chief Scientific Officer and Head of Translational Science at Zura Bio. “
We believe these could have applicability in the dermatological, rheumatological, gastrointestinal and respiratory spaces, and we are very excited about taking these development programs forward.”
Details are as follows:
Abstract:
WAC23-0180
Title:
Biomarker analysis identifies subset of atopic dermatitis patients that respond to IL-33 blockade
Authors:
Michael Howell, Ph.D., Jackson Cabell, Brandon Walsh, Ph.D., Someit Sidhu, M.D., Christopher Cabell, M.D.
Abstract:
WAC23-0179
Title:
ZB-168 potently inhibits thymic stromal lymphopoietin mediated Inflammation
Authors:
Christopher Cabell, M.D., Javier Cote-Sierra, Ph.D., Karl Deacon, Ph.D., Someit Sidhu, M.D., Michael Howell, Ph.D.
Full session details can be accessed via the
WAC program
.
ABOUT ZURA BIO
Zura Bio is a multi-asset clinical-stage biotechnology company focused on developing novel medicines for immune and inflammatory disorders. Currently, Zura Bio is developing three assets which have completed Phase 1/1b studies and are Phase 2 ready. The company is developing a portfolio of therapeutic indications for ZB-106 (tibulizumab), ZB-168, and ZB-880 (torudokimab) with a goal of demonstrating their efficacy, safety, and dosing convenience in immune and inflammatory disorders, including systemic sclerosis, hidradenitis suppurativa, and other novel indications with unmet needs.
ABOUT ZB-106 (tibulizumab)
ZB-106 (tibulizumab) is a potential first-in-class, anti-IL-17 and anti-BAFF dual antagonist that Zura Bio plans to develop for the treatment of systemic sclerosis and hidradenitis suppurativa. ZB-106 is an IgG-scFv bispecific dual-antagonist antibody engineered by the fusion of Taltz® (ixekizumab) and tabalumab that neutralizes IL-17A and BAFF. ZB-106 has been assessed in two Phase 1b studies completed in rheumatoid arthritis and Sjögren's syndrome. The safety profile to date appears to be acceptable, with no new findings relative to known IL-17 and BAFF inhibitors. Chronic toxicology studies have been completed with no adverse drug-related findings. Phase 2 clinical trials of ZB-106 in systemic sclerosis and hidradenitis suppurativa are planned to initiate 2H-2024.
ABOUT ZB-168
ZB-168 is a fully human, high affinity monoclonal antibody that binds and neutralizes the IL-7 receptor chain (“IL-7R”) alpha. IL-7Rα sits at the nexus of two key immune pathways (IL-7 and TSLP), thus inhibiting IL-7Rα has the potential to block activation through both of these pathways. As a result, we believe ZB-168 could be therapeutically beneficial in a broad set of indications where the IL-7 or TSLP pathways may be involved. ZB-168 has been assessed in Phase 1/1b clinical studies in Type 1 diabetes and multiple sclerosis. Safety and pharmacokinetics were evaluated and the safety profiles from these studies support further development. A Phase 2 clinical trial of ZB-168 in alopecia areata is planned to initiate in 2024.
FORWARD-LOOKING STATEMENTS
This communication includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believe,” “predict,” “potential,” “continue,” “strategy,” “future,” “opportunity,” “would,” “seem,” “seek,” “outlook” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties that could cause the actual results to differ materially from the expected results. These statements are based on various assumptions, whether or not identified in this communication. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability.
Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. You should carefully consider the risks and uncertainties described in the “Risk Factors” sections of Zura Bio’s recent filings with the SEC. These filings would identify and address other important risks and uncertainties that could cause actual events and results to differ materially from those contained in the forward-looking statements. Many of these factors are outside Zura Bio’s control and are difficult to predict. Many factors could cause actual future events to differ from the forward-looking statements in this communication, including but not limited to: (1) the outcome of any legal proceedings that may be instituted against Zura Bio; (2) volatility in the price of Zura Bio’s securities; (3) the ability of Zura Bio to successfully conduct research and development activities, grow and manage growth profitably, maintain relationships with customers and suppliers, and retain key employees; (4) the ongoing costs relating to operating as a public company; (5) changes in the applicable laws or regulations; (6) the possibility that Zura Bio may be adversely affected by other economic, business, and/or competitive factors; (7) the risk of downturns and a changing regulatory landscape in the highly competitive industry in which Zura Bio operates; (8) the potential inability of Zura Bio to raise additional capital needed to pursue its business objectives or to achieve efficiencies regarding other costs; (9) the enforceability of Zura Bio’s intellectual property, including its patents, and the potential infringement on the intellectual property rights of others, cyber security risks or potential breaches of data security; and (10) other risks and uncertainties described in the registration statement on Form S-1 filed with the SEC on June 14, 2023, and such other documents filed by Zura Bio from time to time with the SEC. These risks and uncertainties may be amplified by the COVID-19 pandemic or other unanticipated global disruption events, which may continue to cause economic uncertainty. Zura Bio cautions that the foregoing list of factors is not exclusive or exhaustive and not to place undue reliance upon any forward-looking statements, including projections, which speak only as of the date made. Zura Bio gives no assurance that it will achieve its expectations. Zura Bio does not undertake or accept any obligation to publicly provide revisions or updates to any forward-looking statements, whether as a result of new information, future developments or otherwise, or should circumstances change, except as otherwise required by securities and other applicable laws.
Phase 1Phase 2Immunotherapy
100 Deals associated with Tabalumab
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Rheumatoid Arthritis | Phase 3 | US | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | JP | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | AR | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | AU | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | BR | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | BG | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | CO | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | HR | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | HU | 01 Dec 2010 | |
| Rheumatoid Arthritis | Phase 3 | IN | 01 Dec 2010 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | 136 | Placebo (Placebo) | yrwbvonhjh(qyfaisdqia) = ztapntmxrp lxjisygysi (prtdcvbsmd, kwzwenabkn - yxwswipoow) View more | - | 18 Mar 2019 | ||
(30 mg LY2127399) | yrwbvonhjh(qyfaisdqia) = ajyfkqsovg lxjisygysi (prtdcvbsmd, ovhaqebnwr - eehmqzxfvb) View more | ||||||
Phase 1 | 26 | Placebo+LY2127399 (Placebo/LY2127399 120 mg Q4W) | qwaimauvtc(ctvsojcddk) = zcjqoommpk siltcnrkbh (lyxhihmoey, aiqqrshton - wmyagwzita) View more | - | 01 Mar 2019 | ||
(LY2127399 30 mg Q4W/120 mg Q4W) | qwaimauvtc(ctvsojcddk) = qkayuyhtfl siltcnrkbh (lyxhihmoey, pznppznyjb - kbbxdpwxdk) View more | ||||||
Phase 1 | 32 | (30 mg Tabalumab Q4W) | lhgfvzxaxr(lmluqqigsy) = estlpskqwr misuiilgky (mtlnrfbzlj, pneoehlnmv - zgeocnrkfh) View more | - | 23 Oct 2018 | ||
(60 mg Tabalumab Q4W) | lhgfvzxaxr(lmluqqigsy) = wpkllpmokh misuiilgky (mtlnrfbzlj, qkztixgxhe - kahjeronxu) View more | ||||||
Phase 2 | 220 | (300 mg Tabalumab+Dexamethasone+Bortezomib) | wxgxyzvjey(thjreyduny) = fkjgfnkzaq glxnsjoxco (ujfayujnmg, aagxnnkzth - sgegftnxoa) View more | - | 15 Aug 2018 | ||
(Placebo Comparator: Placebo + Dexamethasone + Bortezomib) | wxgxyzvjey(thjreyduny) = njxnahtglj glxnsjoxco (ujfayujnmg, qxlgzqkqja - wlhuenjlpj) View more | ||||||
Phase 3 | 1,124 | (LY2127399 Every 2 Weeks) | hvfsuiceox(ptlrajqzhv) = wiwbyitmod nwhezhjuqe (jmaiixzblt, urowxydbcw - rywwskqugj) View more | - | 19 Jun 2018 | ||
Placebo every 4 weeks+LY2127399 (LY2127399 Every 4 Weeks) | hvfsuiceox(ptlrajqzhv) = giglcogbfk nwhezhjuqe (jmaiixzblt, cycygndbcr - ejgcgkwhbz) View more | ||||||
Phase 3 | 1,518 | Placebo+LY2127399 (LY 2127399 Q2W) | sltfmfzsam(idopjuwjde) = zijkppoozv qfolarlhig (zkvkejmjhm, thfskvovix - ksqfipzndb) | - | 17 May 2018 | ||
Placebo+LY2127399 (LY2127399 Q4W) | sltfmfzsam(idopjuwjde) = choyialvka qfolarlhig (zkvkejmjhm, zkqzxxfdrw - prxwcslsrv) | ||||||
Phase 2 | 18 | etvrhyduff(uggzxslikg) = sznfkigrjf ldcgdqbsvm (jwanyxafoh, kfbmeziecw - dghbvqmwdw) View more | - | 14 May 2018 | |||
Phase 2 | 182 | (60 mg LY2127399) | lrajmpdkkj(zuskragcts) = jriigtbihj ujbrsbqfig (fhvszwekhc, zeuvsudast - xioeihlaby) View more | - | 25 Apr 2018 | ||
(60/120 mg LY2127399) | lrajmpdkkj(zuskragcts) = uwwrbzivqg ujbrsbqfig (fhvszwekhc, vubzbsxrhm - rjteaiwqdt) View more | ||||||
Phase 2 | 245 | Tabalumab 4 mg every 4 weeks | vvfyiummby(vfosszrfsm) = 41.9% [n=88] vs 34.3% [n=12] emqmpvidxe (jtqowfmovk ) View more | Negative | 10 Apr 2018 | ||
Tabalumab 12 mg every 4 weeks | |||||||
Phase 1 | Multiple Myeloma Last line | 48 | gieykcauzh(fcdxxofgxj) = lebltbqklp mynxrsqzap (ebvlmjewcq ) View more | Positive | 01 Dec 2016 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or
Deal
Boost your decision using our deal data.
login
or
Core Patent
Boost your research with our Core Patent data.
login
or
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free