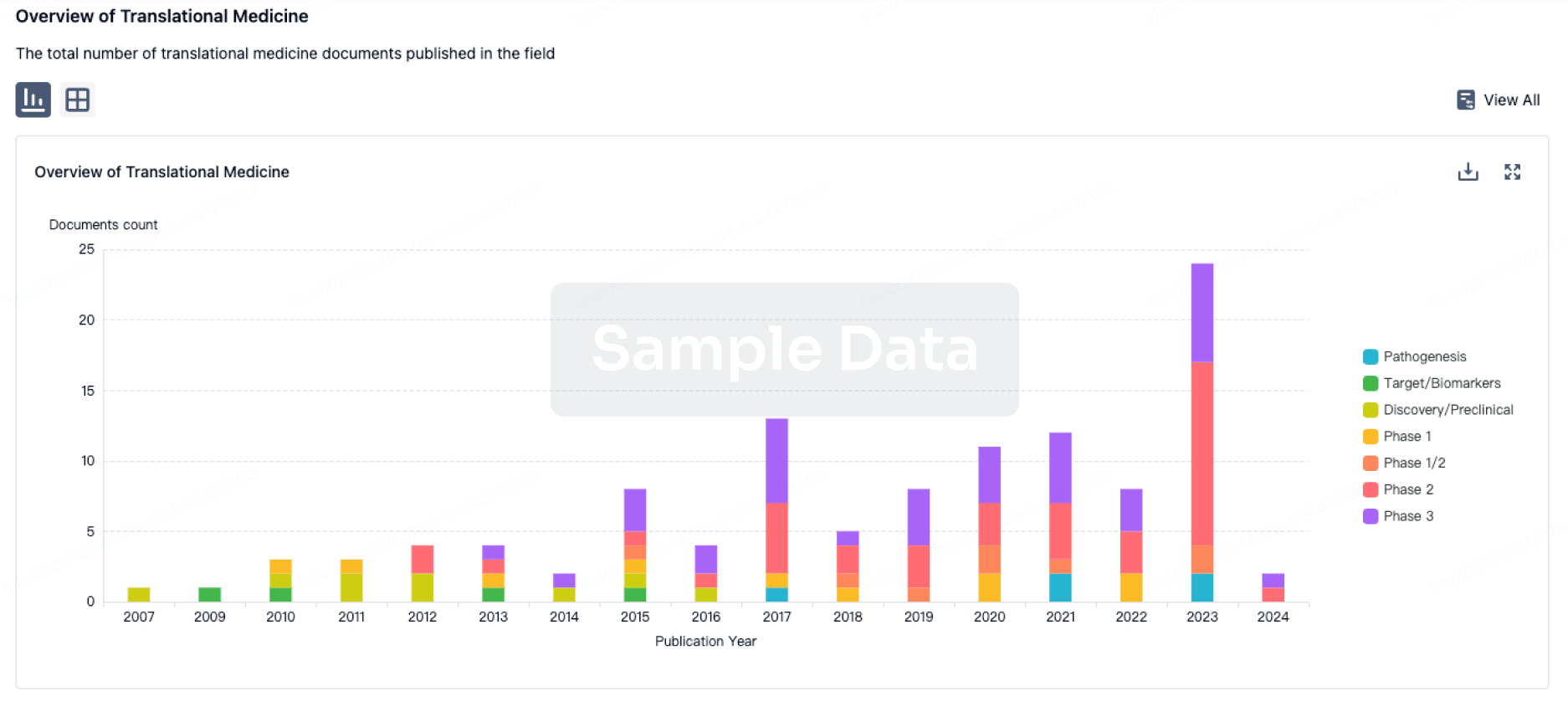
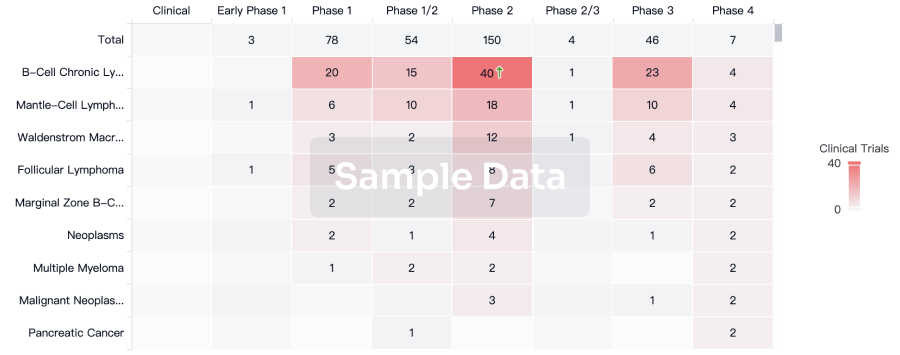
AstraZeneca will share new data across its Vaccines & Immune Therapies portfolio at the 2025 Congress of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID Global 2025) in Vienna, Austria, from 11-15 April 2025. With 13 abstracts, including two oral presentations and two late-breaking poster presentations, the Company will highlight progress in advancing novel immunisations against bacterial and viral infectious diseases and share real-world evidence showing the burden of respiratory viral infections and the continued need for protection.
Benjamin Moutier, Senior Vice President, Vaccines & Immune Therapies, AstraZeneca said: “AstraZeneca is building an innovative portfolio of antibodies and vaccines to tackle two of the top global public health threats – serious bacterial infections and respiratory viral infections. At ESCMID, we’ll share data on three novel monoclonal antibodies targeting high-priority pathogens, highlighting our ambition to redefine care for serious bacterial infections. In addition, we will present 20 years of effectiveness data for Fluenz/FluMist, as well as real-world data highlighting the ongoing burden of RSV and hMPV, two leading causes of respiratory illnesses.”
Advancing potential new options for serious bacterial infection prevention and treatment
Serious bacterial infections pose significant and growing threats to global health, linked to an estimated 7.7 million deaths a year globally, leading to increasing morbidity and mortality for patients and a substantial burden on health systems.1 At ESCMID, the Company will share preclinical data on investigational monoclonal antibodies (mAbs) targeting three of the top five pathogens identified as urgent risks by the Centers for Disease Control and Prevention:
Staphylococcus aureus (S. aureus): Oral presentation on in vivo expression of an mRNA-encoded multi-mechanistic mAb combination against S. aureus. There are millions of methicillin-resistant S. aureus (MRSA) infections annually worldwide, and MRSA is resistant to many types of antibiotics, making treatment highly complex. A pathogen-specific treatment strategy with mAbs targeting some S. aureus virulence factors represents an alternative to broad spectrum antibiotics.2 Pseudomonas aeruginosa (P. aeruginosa): Preclinical data showing that gremubamab, an anti-P. aeruginosa bispecific human mAb, may enhance neutrophil-mediated killing of P. aeruginosa in bronchiectasis patients. P. aeruginosa is a pathogen that colonizes one-third of patients with bronchiectasis, a chronic lung condition impacting about one million people globally and drives significant disease morbidity and mortality.3 Klebsiella pneumoniae (K. pneumoniae): Research on the development and characterisation of bispecific antibodies that protect against bacteraemia and pneumonia from the major serotypes of antibiotic-resistant K. pneumoniae in mouse models. Infections caused by K. pneumoniae are responsible for more than 700,000 yearly deaths worldwide.4
Real-world data examining the impact of respiratory infections
AstraZeneca is presenting 20 years of real-world data on Fluenz/FluMist (live attenuated influenza vaccines, LAIV), recently approved in the U.S. as the only vaccine for self- or caregiver administration for the prevention of influenza, demonstrating long-term effectiveness comparable to that of inactivated (IIV) influenza vaccines in children.5
The Company will also present two late-breaking posters featuring real-world studies in patients with haematological malignancies who are highly susceptible to severe respiratory infections. The studies show that outcomes from human metapneumovirus (hMPV) and respiratory syncytial virus (RSV) were similar to those from influenza and COVID-19, highlighting the need for development of novel options for preventing disease caused by hMPV and RSV.6,7
Retrospective analyses from Valencia, Spain, where there is systematic testing for multiple respiratory viruses, highlight the impact of RSV and influenza on severe disease and mortality in older adults throughout the COVID-19 pandemic, and suggest a potentially greater proportion of mechanical ventilation use and in-hospital death from RSV than influenza in adolescents and adults.8,9 Additional real-world data from France highlight the extensive healthcare resource utilisation due to hMPV and RSV and underline the need for routine testing for hMPV to accurately assess its true burden.10
Additionally, data being presented by our partner, Sanofi, confirm the significant public health impact and real-world effectiveness of Beyfortus in reducing RSV disease and hospitalisations in infants.11
Furthering Research in COVID-19 Prevention
Data on AZD6563, an investigational novel COVID-19 mRNA vaccine-like particle (VLP) vaccine, demonstrating that a low dose may elicit equivalent cell-mediated immunity to a licensed mRNA vaccine will also be shared.12
Additionally, data will be presented increasing confidence in a SARS-CoV-2 variant-agnostic threshold which could be used as a surrogate endpoint for benchmarking expected efficacy of novel mAbs for the prevention of COVID-19 against emerging variants.13
Key AstraZeneca presentations during ESCMID 2025
Abstract Title
Presentation Details
Abstract Title
Early Science: Serious Bacterial Infections and Respiratory Viral Infections
Abstract Title
In vivo expression of an mRNA encoded multi-mechanistic mAb combination against Staphylococcus aureus and protection in disease model
Presentation Details
Oral Presentation
Session Title: Around the Lab in 80 Ways: A Journey Through Innovative Therapeutics
Date: Saturday 12 April 2025
Time: 11 AM – 12 PM CEST
Location: Hall 13
Abstract Title
Low dose of a novel SARS-COV-2 mRNA VLP vaccine elicits equivalent cell-mediated immunity to a licensed mRNA vaccine
Presentation Details
Poster Presentation
Session Title: COVID-19 Date: Saturday 12 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Abstract Title
A Bispecific Monoclonal Antibody Targeting Psl and PcrV Enhances Bronchiectasis Patient Neutrophil-Mediated Killing Of Pseudomonas aeruginosa
Presentation Details
Poster Presentation
Session Title: Drug Discovery and New Compounds Mechanisms of Action & Spectrum, Preclinical Data & Basic Pharmacology
Date: Sunday 13 April 2025
Time: 12 PM – 1:30 PM CEST
Location: Poster Area in Hall D
Abstract Title
Broad coverage of antibiotic-resistant Klebsiella pneumoniae by multi-specific antibodies against the lipopolysaccharide O-antigen
Presentation Details
Poster Presentation
Session Title: Drug Discovery and New Compounds Mechanisms of Action & Spectrum, Preclinical Data & Basic Pharmacology
Date: Sunday 13 April 2025
Time: 12 PM – 1:30 PM CEST
Location: Poster Area in Hall D
Abstract Title
Real-World Evidence: Respiratory Infections
Abstract Title
Comparative outcomes of hMPV, influenza, and SARS-CoV-2 infections in haematologic malignancy patients: A matched cohort analysis
Presentation Details
Late-Breaking Poster Presentation
Session Title: Outbreaks and Public Health Emergencies Date: Tuesday 15 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Abstract Title
Matched cohort study comparing the impact of RSV, influenza, and SARS-CoV-2 infections in patients with haematological malignancies
Presentation Details
Late-Breaking Poster Presentation
Session Title: Outbreaks and Public Health Emergencies Date: Tuesday 15 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Abstract Title
Similar proportions and temporal patterns of influenza, RSV, and hMPV among ESKD patients tested between 2020-2023
Presentation Details
Poster Presentation
Session Title: Influenza and Respiratory Viruses Date: Saturday 12 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Abstract Title
Characterization of hMPV and RSV cases in the French national hospital claims database during the 2022-2023 season
Presentation Details
Poster Presentation
Session Title: Influenza and Respiratory Viruses Date: Saturday 12 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Abstract Title
Shifting viral landscapes: the impact of SARS-CoV-2 on hospitalization, ICU admission, and mortality in respiratory infections among older adults in Valencia, Spain
Presentation Details
Poster Presentation
Session Title: Influenza and Respiratory Viruses
Date: Saturday 12 April 2025
Time: 12 PM – 1:30 PM CEST
Location: Poster Area in Hall D
Abstract Title
Respiratory syncytial virus (RSV)-associated hospitalised influenza-like illness (ILI) and clinical progression in adolescents and adults in Valencia, Spain, 2014/2015-2019/2020: the VALSARI study
Presentation Details
Poster Presentation
Session Title: Influenza and Respiratory Viruses
Date: Saturday 12 April 2025
Time: 12 PM – 1:30 PM CEST
Location: Poster Area in Hall D
Abstract Title
Real-world effectiveness of live attenuated (LAIV) and inactivated (IIV) influenza vaccines in children: a systematic literature review (SLR) and network meta-analysis (NMA)
Presentation Details
Poster Presentation
Session Title: Influenza and Respiratory Viruses Date: Saturday 12 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Abstract Title
COVID-19 mAbs
Abstract Title
Pharmacokinetics and immunogenicity of AZD7442 (Tixagevimab/Cilgavimab) in hospitalised COVID-19 patients: results from the DisCoVeRy trial
Presentation Details
Oral Presentation
Session Title: New developments in prophylaxis and treatment of COVID-19
Date: Saturday 12 April 2025
Time: 11:00
Location: Hall 6
Abstract Title
A threshold of protection model for monoclonal antibody pre-exposure prophylaxis against COVID-19 enabling rapid development for future SARS-CoV-2 variants
Presentation Details
Poster Presentation
Session Title: COVID-19 Date: Saturday 12 April 2025 Time: 12 PM – 1:30 PM CEST Location: Poster Area in Hall D
Notes
AstraZeneca AstraZeneca (LSE/STO/Nasdaq: AZN) is a global, science-led biopharmaceutical company that focuses on the discovery, development, and commercialisation of prescription medicines in Oncology, Rare Diseases, and BioPharmaceuticals, including Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. Based in Cambridge, UK, AstraZeneca's innovative medicines are sold in more than 125 countries and used by millions of patients worldwide. Please visit astrazeneca.com and follow the Company on social media @AstraZeneca.
Contacts
For details on how to contact the Investor Relations Team, please click here. For Media contacts, click here.
References
1. GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022 Dec 17;400(10369):2221-2248.
2. Tkaczyk C, Newton M et al. In vivo expression of an mRNA encoded multi-mechanistic mAb combination against Staphylococcus aureus and protection in disease model. Oral Presentation. ESCMID Global 2025; Vienna, Austria. 2025.
3. Long MB, Gilmour A et al. A bispecific monoclonal antibody targeting Psl and PcrV enhances bronchiectasis patient neutrophil-mediated killing of Pseudomonas aeruginosa. Poster Presentation #P3642. ESCMID Global 2025; Vienna, Austria. 2025.
4. Doyle M, Smith A et al. Broad coverage of antibiotic-resistant Klebsiella pneumoniae by multi-specific antibodies against the lipopolysaccharide O-antigen. Poster Presentation #P2377. ESCMID Global 2025; Vienna, Austria. 2025.
5. Stuurman AL, Enxing J et al. Real-world effectiveness of live attenuated (LAIV) and inactivated (IIV) influenza vaccines in children: a systematic literature review (SLR) and network meta-analysis (NMA). Poster Presentation #P0120. ESCMID Global 2025; Vienna, Austria. 2025.
6. Salmanton-Garcia J, Marchesi F et al. Comparative outcomes of hMPV, influenza, and SARS-CoV-2 infections in haematologic malignancy patients: a matched cohort analysis. Poster Presentation #P5067. ESCMID Global 2025; Vienna, Austria. 2025.
7. Marchesi F, Salmanton-Garcia J et al. Matched cohort study comparing the impact of RSV, influenza, and SARS-CoV-2 infections in patients with haematologic malignancies. Poster Presentation #P5066. ESCMID Global 2025; Vienna, Austria. 2025.
8. Mira-Iglesias A, Herring T et al. Respiratory syncytial virus (RSV)-associated hospitalisations and clinical progression in adolescents and adults in Valencia, Spain, 2014/2015–2019/2020: the VALSARI study. Poster Presentation #P0131. ESCMID Global 2025; Vienna, Austria. 2025.
9. Mira-Iglesias A, Herring T et al. Respiratory syncytial virus (RSV)-associated hospitalisations and clinical progression in adolescents and adults in Valencia, Spain, 2014/2015–2019/2020: the VALSARI study. Poster Presentation #P0132. ESCMID Global 2025; Vienna, Austria. 2025.
10. Binh Luong Nguyen L, et al. Characterisation of hMPV and RSV hospitalisations in the French national hospital claims database during the 2022-2023 season. Poster Presentation #P0183. ESCMID Global 2025; Vienna, Austria. 2025.
11. Mallah N, Pardo-Seco J, et al. Effectiveness of universal prophylaxis with nirsevimab for prevention of infant hospitalisations due to respiratory syncytial virus : full 2023-24 season analysis of the NIRSE-GAL study. Poster Presentation #P0124. ESCMID Global 2025; Vienna, Austria. 2025.
12. Swanson PA II, Padilla M et al. Low dosage of a novel SARS-COV-2 mRNA VLP vaccine elicits equivalent cell-mediated immunity to a licensed mRNA vaccine. Poster Presentation # P0482. ESCMID Global 2025; Vienna, Austria. 2025.
13. Edge R, Matthews S et al. A validated threshold of protection model for monoclonal antibody pre-exposure prophylaxis against COVID-19 enabling rapid development for future SARS-CoV-2 variants. Poster Presentation #P0448. ESCMID Global 2025; Vienna, Austria. 2025.
Corporate and financial