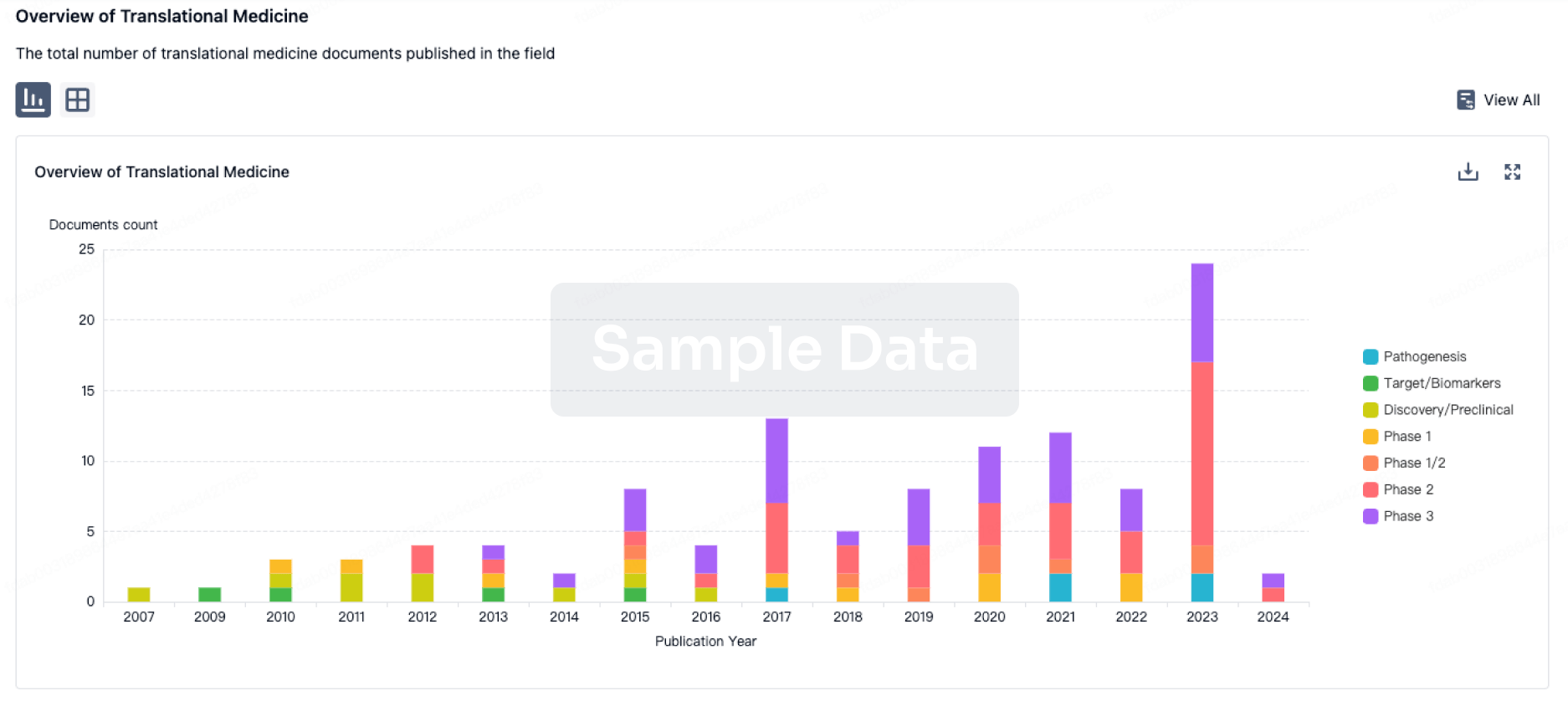
AB Science announced the publication of the masitinib pivotal phase 3 clinical trial in Alzheimer’s Disease in the journal Alzheimer's Research & Therapy
PUBLICATION OF THE MASITINIB PIVOTAL PHASE 3 CLINICAL TRIAL IN ALZHEIMER'S DISEASE IN THE JOURNAL ALZHEIMER'S RESEARCH & THERAPY
AB Science SA (Euronext - FR0010557264 - AB) today announced publication of results from its positive pivotal phase 3 trial (AB09004) of masitinib in mild-to-moderate Alzheimer’s disease (AD) in the renowned international peer-reviewed journal Alzheimer's Research & Therapy [1].
This article, titled ‘Masitinib for mild-to-moderate Alzheimer’s disease: results from a randomized, placebo-controlled, phase 3, clinical trial’ is freely accessible online from the journal website: https://alzres-biomedcentral-com.libproxy1.nus.edu.sg/articles/10.1186/s13195-023-01169-x
Professor Bruno Dubois, director of the Institute of Memory and Alzheimer’s Disease at the Pitié Salpêtrière Hospital in Paris, France, and senior author of this article commented: “This publication provides the first clinical evidence that targeting innate immune cells is an effective strategy for the treatment of mild-to-moderate dementia due to probable Alzheimer’s disease. Results showed that masitinib at 4.5 mg/kg/day can benefit patients by significantly slowing cognitive deterioration relative to placebo. There are very limited treatment options for patients with mild-to-moderate Alzheimer’s disease and to date no approved disease modifying therapy encompasses this later-stage Alzheimer’s disease population. I am therefore excited to continuing the development of masitinib in its confirmatory Phase 3 study (AB21004), with the anticipation that it could benefit this difficult to treat population.”
Professor Olivier Hermine, MD, President of the Scientific Committee of AB Science and member of the Académie des Sciences in France said, “Masitinib is a highly innovative drug for Alzheimer’s disease because unlike the majority of drug development research in this indication, masitinib targets the brain’s innate immune system, including mast cells. The positioning of masitinib as a treatment of mild and moderate Alzheimer’s disease is also different from other drugs, for example, anti-amyloid antibodies such as aduhelm and lecanemab target very mild dementia, prodromal or asymptomatic Alzheimer’s disease. Remarkably, masitinib has now demonstrated neuroprotective benefits in three challenging neurodegenerative disorders; namely mild-to-moderate Alzheimer’s disease [1], amyotrophic lateral sclerosis (ALS) [2,3], and progressive forms of multiple sclerosis [4]. Modulation of the neuroimmune system could therefore be a valid strategy across a broad range of neurodegenerative diseases, with masitinib now uniquely positioned to realize this therapeutic potential.”
Study AB09004 met its primary analysis endpoint, demonstrating that masitinib administered at 4.5 mg/kg/day significantly slowed cognitive deterioration relative to placebo with a manageable safety profile.
Results showed that masitinib can generate a significant treatment effect relative to placebo in the primary endpoint of ADAS-Cog, an instrument that measures the effect on cognition and memory. Specifically, masitinib 4.5 mg/kg/day (n=182) showed significant benefit relative to placebo (n=176), with a change in ADAS-cog from baseline of -1.46 (representing an overall improvement in cognition) versus +0.69 (representing increased cognitive deterioration), respectively; a corresponding ADAS-cog between-group difference of -2.15 (97.5%CI [-3.48, -0.81]), p=0.0003. This change is considered clinically meaningful, especially when considering its administration on a background of cholinesterase inhibitors and memantine, a 2-point change being consistent with published recommendations [5] and benchmark ADAS-Cog benefit according to well-established therapies [6-8].
It was also seen that masitinib generated a non-significant trend towards improved overall function relative to placebo as measured by the ADCS-ADL score, an instrument that assesses self-care and activities of daily living. Specifically, masitinib 4.5 mg/kg/day showed a change in ADCS-ADL from baseline of +1.01 (representing an overall functional improvement) versus -0.81 for placebo (representing increased functional deterioration); a corresponding ADCS-ADL between-group difference of +1.82 (97.5%CI [(-0.15, 3.79]), p=0.038.
The safety of masitinib as an adjunct to cholinesterase inhibitor and/or memantine was acceptable and consistent with its known tolerability profile. It is noteworthy that this result is in the context of a relative elderly population (average age of about 73 years) with comorbidities.
About the confirmatory Phase 3 trial (AB21004)
The objective of study AB21004 is to confirm results from the first phase 2B/3 study, AB09004. Study AB21004 has recently received an Investigational New Drug (IND) approval letter from the FDA, with similar authorizations having also been received from several European countries, including the French Medicine Agency (ANSM). This study will enroll 600 patients with confirmed clinical diagnosis of mild and moderate Alzheimer's disease, corresponding to a Mini Mental State Examination (MMSE) score of between 14 to 25, inclusive. The primary objective of the study will be to evaluate the effect of masitinib 4.5 mg/kg/day, administered as an add-on therapy to standard of care (cholinesterase inhibitors and/or memantine), on absolute change from baseline in ADCS-ADL score and in ADAS-Cog-11.
About masitinib’s mechanism of action in Alzheimer’s disease
Masitinib (AB1010) is an oral tyrosine kinase inhibitor that has demonstrated neuroprotective action in neurodegenerative diseases via inhibition of mast cell and microglia/macrophage activity, possibly by switching the neuroimmune system from a neurotoxic state towards a neuroprotective state through remodeling of the neuronal microenvironment. Mast cells, macrophages and microglia are types of innate immune cells that are present in the central nervous system, and for which there is a growing body of evidence implicating them in the pathophysiology of neurodegenerative diseases such as Alzheimer’s disease, progressive forms of multiple sclerosis and amyotrophic lateral sclerosis (ALS).
The rationale to use masitinib in Alzheimer’s disease patients is supported by preclinical evidence demonstrating that the pharmacological action of masitinib in mast cells can restore normal spatial learning performance in a mouse model of Alzheimer’s disease and promotes recovery of synaptic markers [9].
Despite decades of extensive research, the overwhelming majority of human trials (predominantly testing amyloid-based therapeutics) have failed to demonstrate clinical efficacy. This underscores a need for innovative, non-amyloid based approaches, including therapies that modulate the neuroimmune response in Alzheimer’s disease, which has been implicated in the pathophysiology of the disease [10–14].
References:
[1] Dubois B, López‑Arrieta J, Lipschitz S, et al. Masitinib for mild-to-moderate Alzheimer’s disease: results from a randomized, placebo-controlled, phase 3, clinical trial. Alzheimer’s Research & Therapy. 2023 https://doi-org.libproxy1.nus.edu.sg/10.1186/s13195-023-01169-x
[2] Mora JS, Genge A, Chio A, et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(1-2):5-14. doi:10.1080/21678421.2019.1632346
[3] Mora JS, Bradley WG, Chaverri D, et al. Long-term survival analysis of masitinib in amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2021;14:17562864211030365. doi:10.1177/17562864211030365
[4] Vermersch P, Brieva-Ruiz L, Fox RJ, et al. Efficacy and Safety of Masitinib in Progressive Forms of Multiple Sclerosis: A Randomized, Phase 3, Clinical Trial. Neurol Neuroimmunol Neuroinflamm 2022;9:e1148. doi:10.1212/NXI.0000000000001148
[5] Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G; European Task Force Group. Lancet Neurol. 2008;7(5):436-450.
[6] Birks JS, Harvey RJ. Cochrane Database Syst Rev. 2018;6(6):CD001190. Published 2018 Jun 18.
[7] Birks JS, Chong LY, Grimley Evans J. Cochrane Database Syst Rev. 2015;9(9):CD001191.
[8] Birks J. Cochrane Database Syst Rev. 2006;2006(1):CD005593. Published 2006 Jan 25.
[9] Li T, Martin E, Abada YS, et al. J Alzheimers Dis. 2020;76(4):1339-1345.
[10] Long JM, Holtzman DM. Cell. 2019;179(2):312-339
[11] Sandhu JK, Kulka M. Int J Mol Sci. 2021;22(3):1093.
[12] Klegeris A. Front. Drug Chem. Clin. Res. 2020;3:1–4
[13] Tchessalova D, Posillico CK, Tronson NC. Front Syst Neurosci. 2018;12:39.
[14] Li JW, Zong Y, Cao XP, Tan L, Tan L. Ann Transl Med. 2018;6(10):176.
About AB Science
Founded in 2001, AB Science is a pharmaceutical company specializing in the research, development and commercialization of protein kinase inhibitors (PKIs), a class of targeted proteins whose action are key in signaling pathways within cells. Our programs target only diseases with high unmet medical needs, often lethal with short term survival or rare or refractory to previous line of treatment.
AB Science has developed a proprietary portfolio of molecules and the Company’s lead compound, masitinib, has already been registered for veterinary medicine and is developed in human medicine in oncology, neurological diseases, inflammatory diseases and viral diseases. The company is headquartered in Paris, France, and listed on Euronext Paris (ticker: AB).