Request Demo
Last update 08 May 2025
Thymosin beta-4
Last update 08 May 2025
Overview
Basic Info
Drug Type Synthetic peptide |
Synonyms Fx peptide, Thymosin B4, Thymosin beta 4 + [15] |
Target |
Action modulators |
Mechanism thymosin beta 4 modulators(Thymosin Beta-4 modulators) |
Therapeutic Areas |
Active Indication |
Inactive Indication |
Originator Organization |
Active Organization |
Inactive Organization |
Drug Highest PhasePhase 3 |
First Approval Date- |
RegulationSpecial Review Project (China), Orphan Drug (United States) |
Login to view timeline
Structure/Sequence
Sequence Code 8196

Source: *****
Related
17
Clinical Trials associated with Thymosin beta-4CTRI/2025/03/083304
Evaluation of salivary thymosin Beta-4 levels, antimicrobial efficacy and pain perception following platelet rich plasma (PRP) administration during orthodontic therapy - Nil
Start Date17 Jul 2025 |
Sponsor / Collaborator- |
CTIS2022-502697-16-00
SEER-3: A Phase 3, Multi-Center, Randomized, Parallel, Double Masked, Placebo-Controlled Clinical Study to Assess the Safety and Efficacy of 0.1% RGN-259 Ophthalmic Solution for the Treatment of Neurotrophic Keratopathy - SEER-3
Start Date03 Oct 2023 |
Sponsor / Collaborator |
NCT05555589
A Phase 3, Multi-Center, Randomized, Parallel, Double Masked, Placebo-Controlled Clinical Study to Assess the Safety and Efficacy of 0.1% RGN-259 Ophthalmic Solution for the Treatment of Neurotrophic Keratopathy (SEER-2)
The objective of this study is to compare the safety and efficacy of RGN-259 to placebo for the treatment of Neurotrophic Keratopathy (NK)
Start Date11 Apr 2023 |
Sponsor / Collaborator |
100 Clinical Results associated with Thymosin beta-4
Login to view more data
100 Translational Medicine associated with Thymosin beta-4
Login to view more data
100 Patents (Medical) associated with Thymosin beta-4
Login to view more data
268
Literatures (Medical) associated with Thymosin beta-403 Apr 2025·Journal of Pharmacy and Pharmacology
Inhaled exogenous thymosin beta 4 suppresses bleomycin-induced pulmonary fibrosis in mice via TGF-β1 signalling pathway
Article
Author: Xu, Junjie ; Yu, Rui ; Liu, Yanhong ; Liu, Zhichao ; Zhang, Qianyi ; Hu, Enbo ; Mao, Yunyun ; Chen, Li ; Li, Xiaohe ; Yang, Cheng ; Zhai, Yanfang ; Li, Shimeng ; Li, Kai ; Zhou, Honggang ; Chai, Dan
01 Feb 2025·Free Radical Biology and Medicine
Enhancing fat graft survival: thymosin beta-4 facilitates mitochondrial transfer from ADSCs via tunneling nanotubes by upregulating the Rac/F-actin pathway
Article
Author: Wang, Qian ; Lin, Yan ; Zhang, Xiaoyu ; Mu, Dali ; Li, Haoran
01 Nov 2024·Asian Journal of Surgery
Therapeutic potential of thymosin beta 4 in a variety of diseases
Letter
Author: Dai, Qingmei ; Ye, Chaoqun ; Deng, Luyao ; Huang, Huizhi
18
News (Medical) associated with Thymosin beta-424 Aug 2023
Akosua Mireku
@AkosuaMireku1
CyclASol is an eye drop solution for dry eye disease. Credit: Andrii Borodai via Getty Images.
The European Medicines Agency (EMA) accepted Novaliq’s marketing authorisation application for CyclASol (cyclosporine ophthalmic solution) 0.1% as a treatment for
dry eye disease (DED)
.
Free Report
How is the Biopharmaceutical industry evolving?
2021 was a year of continued innovation and change in the Biopharmaceutical industry. As the COVID-19 pandemic continues to take its toll on businesses worldwide, it’s time to look for new ways to create value, prepare for the future, and remain competitive in the ever-changing landscape.
GlobalData’s expansive report examines the business environment and trends that shape the Biopharmaceutical industry. We highlight the most impactful emerging technologies, as well as the industry, regulatory, and macroeconomic factors that influence growth prospects.
Access the report to:
Benchmark the impact of major themes on the Biopharmaceutical industry.
Gain a deeper "on the ground" perspective through exclusive opinions and analysis from industry respondents.
Evaluate the effects of COVID-19 on the sector.
Download the full report to understand what to expect and how to align your strategies for success.
Submit
Country Code *
UK (+44)
USA (+1)
Algeria (+213)
Andorra (+376)
Angola (+244)
Anguilla (+1264)
Antigua & Barbuda (+1268)
Argentina (+54)
Armenia (+374)
Aruba (+297)
Australia (+61)
Austria (+43)
Azerbaijan (+994)
Bahamas (+1242)
Bahrain (+973)
Bangladesh (+880)
Barbados (+1246)
Belarus (+375)
Belgium (+32)
Belize (+501)
Benin (+229)
Bermuda (+1441)
Bhutan (+975)
Bolivia (+591)
Bosnia Herzegovina (+387)
Botswana (+267)
Brazil (+55)
Brunei (+673)
Bulgaria (+359)
Burkina Faso (+226)
Burundi (+257)
Cambodia (+855)
Cameroon (+237)
Canada (+1)
Cape Verde Islands (+238)
Cayman Islands (+1345)
Central African Republic (+236)
Chile (+56)
China (+86)
Colombia (+57)
Comoros (+269)
Congo (+242)
Cook Islands (+682)
Costa Rica (+506)
Croatia (+385)
Cuba (+53)
Cyprus North (+90392)
Cyprus South (+357)
Czech Republic (+42)
Denmark (+45)
Djibouti (+253)
Dominica (+1809)
Dominican Republic (+1809)
Ecuador (+593)
Egypt (+20)
El Salvador (+503)
Equatorial Guinea (+240)
Eritrea (+291)
Estonia (+372)
Ethiopia (+251)
Falkland Islands (+500)
Faroe Islands (+298)
Fiji (+679)
Finland (+358)
France (+33)
French Guiana (+594)
French Polynesia (+689)
Gabon (+241)
Gambia (+220)
Georgia (+7880)
Germany (+49)
Ghana (+233)
Gibraltar (+350)
Greece (+30)
Greenland (+299)
Grenada (+1473)
Guadeloupe (+590)
Guam (+671)
Guatemala (+502)
Guinea (+224)
Guinea - Bissau (+245)
Guyana (+592)
Haiti (+509)
Honduras (+504)
Hong Kong (+852)
Hungary (+36)
Iceland (+354)
India (+91)
Indonesia (+62)
Iran (+98)
Iraq (+964)
Ireland (+353)
Israel (+972)
Italy (+39)
Jamaica (+1876)
Japan (+81)
Jordan (+962)
Kazakhstan (+7)
Kenya (+254)
Kiribati (+686)
Korea North (+850)
Korea South (+82)
Kuwait (+965)
Kyrgyzstan (+996)
Laos (+856)
Latvia (+371)
Lebanon (+961)
Lesotho (+266)
Liberia (+231)
Libya (+218)
Liechtenstein (+417)
Lithuania (+370)
Luxembourg (+352)
Macao (+853)
Macedonia (+389)
Madagascar (+261)
Malawi (+265)
Malaysia (+60)
Maldives (+960)
Mali (+223)
Malta (+356)
Marshall Islands (+692)
Martinique (+596)
Mauritania (+222)
Mayotte (+269)
Mexico (+52)
Micronesia (+691)
Moldova (+373)
Monaco (+377)
Mongolia (+976)
Montserrat (+1664)
Morocco (+212)
Mozambique (+258)
Myanmar (+95)
Namibia (+264)
Nauru (+674)
Nepal (+977)
Netherlands (+31)
New Caledonia (+687)
New Zealand (+64)
Nicaragua (+505)
Niger (+227)
Nigeria (+234)
Niue (+683)
Norfolk Islands (+672)
Northern Marianas (+670)
Norway (+47)
Oman (+968)
Palau (+680)
Panama (+507)
Papua New Guinea (+675)
Paraguay (+595)
Peru (+51)
Philippines (+63)
Poland (+48)
Portugal (+351)
Puerto Rico (+1787)
Qatar (+974)
Reunion (+262)
Romania (+40)
Russia (+7)
Rwanda (+250)
San Marino (+378)
Sao Tome & Principe (+239)
Saudi Arabia (+966)
Senegal (+221)
Serbia (+381)
Seychelles (+248)
Sierra Leone (+232)
Singapore (+65)
Slovak Republic (+421)
Slovenia (+386)
Solomon Islands (+677)
Somalia (+252)
South Africa (+27)
Spain (+34)
Sri Lanka (+94)
St. Helena (+290)
St. Kitts (+1869)
St. Lucia (+1758)
Sudan (+249)
Suriname (+597)
Swaziland (+268)
Sweden (+46)
Switzerland (+41)
Syria (+963)
Taiwan (+886)
Tajikstan (+7)
Thailand (+66)
Togo (+228)
Tonga (+676)
Trinidad & Tobago (+1868)
Tunisia (+216)
Turkey (+90)
Turkmenistan (+7)
Turkmenistan (+993)
Turks & Caicos Islands (+1649)
Tuvalu (+688)
Uganda (+256)
Ukraine (+380)
United Arab Emirates (+971)
Uruguay (+598)
Uzbekistan (+7)
Vanuatu (+678)
Vatican City (+379)
Venezuela (+58)
Vietnam (+84)
Virgin Islands - British (+1284)
Virgin Islands - US (+1340)
Wallis & Futuna (+681)
Yemen (North)(+969)
Yemen (South)(+967)
Zambia (+260)
Zimbabwe (+263)
Country *
UK
USA
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bonaire, Sint Eustatius and Saba
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos Islands
Colombia
Comoros
Congo
Democratic Republic of
the Congo
Cook Islands
Costa Rica
Côte d"Ivoire
Croatia
Cuba
Curaçao
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-Bissau
Guyana
Haiti
Heard Island and McDonald Islands
Holy See
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
North Korea
South Korea
Kuwait
Kyrgyzstan
Lao
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia
Moldova
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Réunion
Romania
Russian Federation
Rwanda
Saint Helena, Ascension and Tristan da Cunha
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan
Tajikistan
Tanzania
Thailand
Timor-Leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
US Minor Outlying Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Vietnam
British Virgin Islands
US Virgin Islands
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Kosovo
By downloading this case study, you acknowledge that GlobalData may share your information with
GlobalData
and that your personal data will be used as described in their
Privacy Policy
Submit
Visit our
Privacy Policy
for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Report.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) will review the MAA, using the centralised procedure for all 27 member states of the European Union.
Recommended Reports
Reports
LOA and PTSR Model - Progesterone in Keratoconjunctivitis Sicca (Dry Eye)
GlobalData
Reports
LOA and PTSR Model - Timbetasin Acetate in Keratoconjunctivitis Sicca (Dry Eye)
GlobalData
View all
Companies Intelligence
Harrow Health Inc
Novaliq GmbH
MAA Co Ltd
DED Limited
C H M P INC
View all
In order to determine the drug’s approval chances, the CHMP will evaluate results from six clinical trials in CyclASol’s clinical development programme. This includes results from the
Phase III ESSENCE-2 trial
(NCT04523129) that showed that the eye drops had a good tolerability profile, with a clinically meaningful effect on 71.6% of participants in the cyclosporine group.
Germany-headquartered Novaliq received
regulatory approval
for CyclASol from the US Food and Drug Administration (FDA) as a DED treatment on 30 May. Marketed under the US brand name, Vevye, the drug became the first and only cyclosporine solution approved for the condition with efficacy demonstrated after four weeks of treatment. In the US and Canada,
Harrow Health
holds the rights to commercialise the DED therapy, as per a
July 2023 deal
.
CyclASol’s mechanism of action works through the binding of cyclosporine to cyclophilin. This complex suppresses a pathological immune response, which prevents ocular inflammation—a major cause of dry eye disease in many patients. The therapy uses Novaliq’s EyeSol technology to avoid the need for water-based excipients. The biotech lists the benefits of this approach as increasing the drug’s bioavailability and providing a longer residual time on the eye amongst other things.
Novaliq’s pipeline currently consists of
five DED products
and seven candidates for other ophthalmologic conditions. The company is also pursuing research for dermatological treatments through a separate entity Dermaliq. The dermatology clinical programme is currently investigating three drug candidates for androgenetic alopecia, psoriasis, and bacterial skin infections, using its proprietary hyliQ drug delivery technology.
In a press release
, Novaliq’s CEO Christian Roesky said: “The acceptance of the EU marketing authorisation application is a key milestone in our global efforts to address high unmet needs to better serve patients suffering from dry eye disease.
“We look forward to collaborating with the CHMP throughout the review process and hope to make CyclASol available for patients in Europe.”
Free Report
How is the Biopharmaceutical industry evolving?
2021 was a year of continued innovation and change in the Biopharmaceutical industry. As the COVID-19 pandemic continues to take its toll on businesses worldwide, it’s time to look for new ways to create value, prepare for the future, and remain competitive in the ever-changing landscape.
GlobalData’s expansive report examines the business environment and trends that shape the Biopharmaceutical industry. We highlight the most impactful emerging technologies, as well as the industry, regulatory, and macroeconomic factors that influence growth prospects.
Access the report to:
Benchmark the impact of major themes on the Biopharmaceutical industry.
Gain a deeper "on the ground" perspective through exclusive opinions and analysis from industry respondents.
Evaluate the effects of COVID-19 on the sector.
Download the full report to understand what to expect and how to align your strategies for success.
By GlobalData
Submit
Country Code *
UK (+44)
USA (+1)
Algeria (+213)
Andorra (+376)
Angola (+244)
Anguilla (+1264)
Antigua & Barbuda (+1268)
Argentina (+54)
Armenia (+374)
Aruba (+297)
Australia (+61)
Austria (+43)
Azerbaijan (+994)
Bahamas (+1242)
Bahrain (+973)
Bangladesh (+880)
Barbados (+1246)
Belarus (+375)
Belgium (+32)
Belize (+501)
Benin (+229)
Bermuda (+1441)
Bhutan (+975)
Bolivia (+591)
Bosnia Herzegovina (+387)
Botswana (+267)
Brazil (+55)
Brunei (+673)
Bulgaria (+359)
Burkina Faso (+226)
Burundi (+257)
Cambodia (+855)
Cameroon (+237)
Canada (+1)
Cape Verde Islands (+238)
Cayman Islands (+1345)
Central African Republic (+236)
Chile (+56)
China (+86)
Colombia (+57)
Comoros (+269)
Congo (+242)
Cook Islands (+682)
Costa Rica (+506)
Croatia (+385)
Cuba (+53)
Cyprus North (+90392)
Cyprus South (+357)
Czech Republic (+42)
Denmark (+45)
Djibouti (+253)
Dominica (+1809)
Dominican Republic (+1809)
Ecuador (+593)
Egypt (+20)
El Salvador (+503)
Equatorial Guinea (+240)
Eritrea (+291)
Estonia (+372)
Ethiopia (+251)
Falkland Islands (+500)
Faroe Islands (+298)
Fiji (+679)
Finland (+358)
France (+33)
French Guiana (+594)
French Polynesia (+689)
Gabon (+241)
Gambia (+220)
Georgia (+7880)
Germany (+49)
Ghana (+233)
Gibraltar (+350)
Greece (+30)
Greenland (+299)
Grenada (+1473)
Guadeloupe (+590)
Guam (+671)
Guatemala (+502)
Guinea (+224)
Guinea - Bissau (+245)
Guyana (+592)
Haiti (+509)
Honduras (+504)
Hong Kong (+852)
Hungary (+36)
Iceland (+354)
India (+91)
Indonesia (+62)
Iran (+98)
Iraq (+964)
Ireland (+353)
Israel (+972)
Italy (+39)
Jamaica (+1876)
Japan (+81)
Jordan (+962)
Kazakhstan (+7)
Kenya (+254)
Kiribati (+686)
Korea North (+850)
Korea South (+82)
Kuwait (+965)
Kyrgyzstan (+996)
Laos (+856)
Latvia (+371)
Lebanon (+961)
Lesotho (+266)
Liberia (+231)
Libya (+218)
Liechtenstein (+417)
Lithuania (+370)
Luxembourg (+352)
Macao (+853)
Macedonia (+389)
Madagascar (+261)
Malawi (+265)
Malaysia (+60)
Maldives (+960)
Mali (+223)
Malta (+356)
Marshall Islands (+692)
Martinique (+596)
Mauritania (+222)
Mayotte (+269)
Mexico (+52)
Micronesia (+691)
Moldova (+373)
Monaco (+377)
Mongolia (+976)
Montserrat (+1664)
Morocco (+212)
Mozambique (+258)
Myanmar (+95)
Namibia (+264)
Nauru (+674)
Nepal (+977)
Netherlands (+31)
New Caledonia (+687)
New Zealand (+64)
Nicaragua (+505)
Niger (+227)
Nigeria (+234)
Niue (+683)
Norfolk Islands (+672)
Northern Marianas (+670)
Norway (+47)
Oman (+968)
Palau (+680)
Panama (+507)
Papua New Guinea (+675)
Paraguay (+595)
Peru (+51)
Philippines (+63)
Poland (+48)
Portugal (+351)
Puerto Rico (+1787)
Qatar (+974)
Reunion (+262)
Romania (+40)
Russia (+7)
Rwanda (+250)
San Marino (+378)
Sao Tome & Principe (+239)
Saudi Arabia (+966)
Senegal (+221)
Serbia (+381)
Seychelles (+248)
Sierra Leone (+232)
Singapore (+65)
Slovak Republic (+421)
Slovenia (+386)
Solomon Islands (+677)
Somalia (+252)
South Africa (+27)
Spain (+34)
Sri Lanka (+94)
St. Helena (+290)
St. Kitts (+1869)
St. Lucia (+1758)
Sudan (+249)
Suriname (+597)
Swaziland (+268)
Sweden (+46)
Switzerland (+41)
Syria (+963)
Taiwan (+886)
Tajikstan (+7)
Thailand (+66)
Togo (+228)
Tonga (+676)
Trinidad & Tobago (+1868)
Tunisia (+216)
Turkey (+90)
Turkmenistan (+7)
Turkmenistan (+993)
Turks & Caicos Islands (+1649)
Tuvalu (+688)
Uganda (+256)
Ukraine (+380)
United Arab Emirates (+971)
Uruguay (+598)
Uzbekistan (+7)
Vanuatu (+678)
Vatican City (+379)
Venezuela (+58)
Vietnam (+84)
Virgin Islands - British (+1284)
Virgin Islands - US (+1340)
Wallis & Futuna (+681)
Yemen (North)(+969)
Yemen (South)(+967)
Zambia (+260)
Zimbabwe (+263)
Country *
UK
USA
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bonaire, Sint Eustatius and Saba
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos Islands
Colombia
Comoros
Congo
Democratic Republic of
the Congo
Cook Islands
Costa Rica
Côte d"Ivoire
Croatia
Cuba
Curaçao
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-Bissau
Guyana
Haiti
Heard Island and McDonald Islands
Holy See
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
North Korea
South Korea
Kuwait
Kyrgyzstan
Lao
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia
Moldova
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Réunion
Romania
Russian Federation
Rwanda
Saint Helena, Ascension and Tristan da Cunha
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan
Tajikistan
Tanzania
Thailand
Timor-Leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
US Minor Outlying Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Vietnam
British Virgin Islands
US Virgin Islands
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Kosovo
By downloading this case study, you acknowledge that GlobalData may share your information with
GlobalData
and that your personal data will be used as described in their
Privacy Policy
Submit
Visit our
Privacy Policy
for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Report.

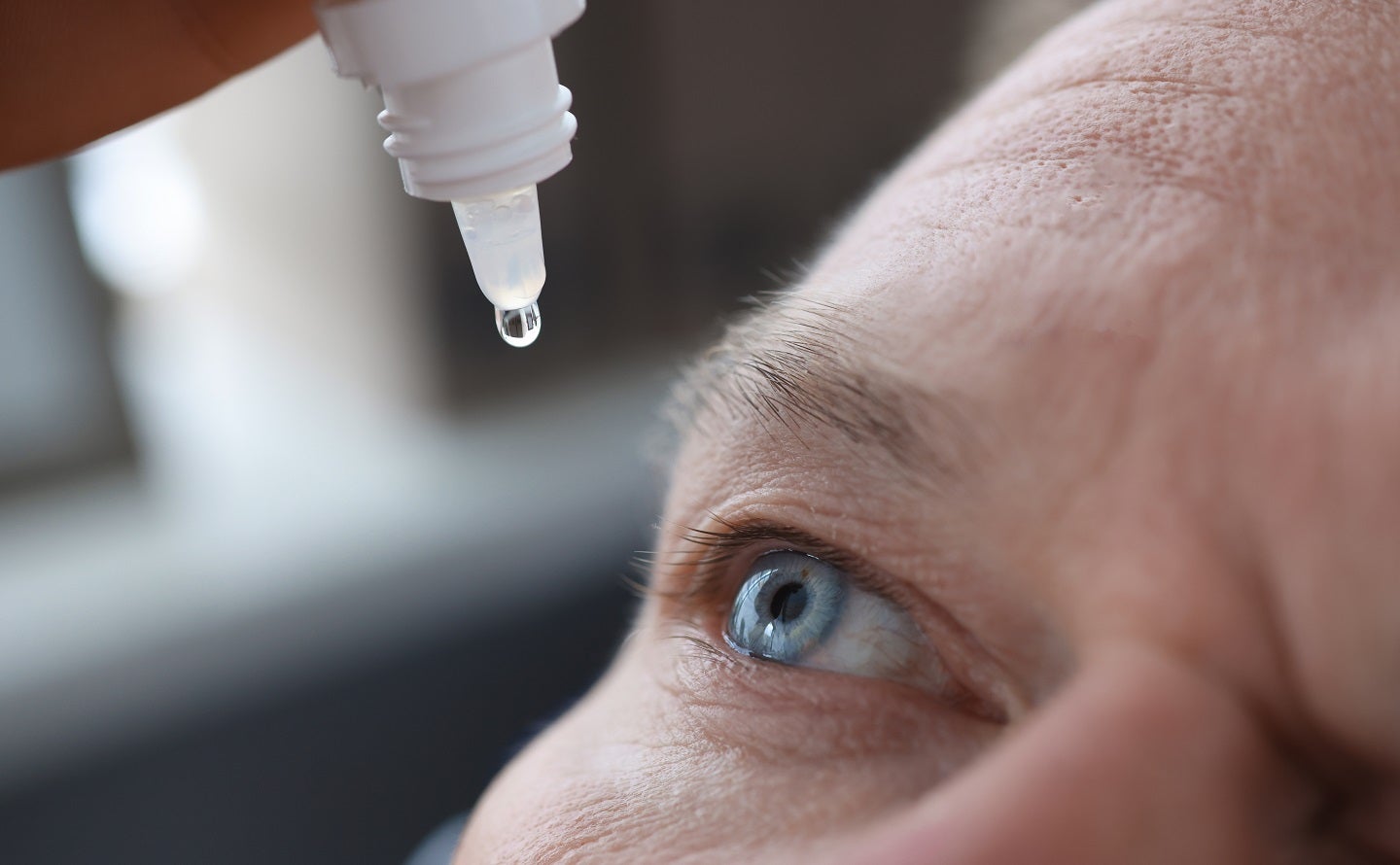
Phase 3Drug ApprovalClinical Result
09 Jun 2023
Novaliq’s VEVYE (cyclosporine ophthalmic solution) 0.1% is indicated to treat the signs and symptoms of dry eye disease. Credit: megaflopp via Shutterstock.com.
Biopharmaceutical company Novaliq has received approval from the
US Food and Drug Administration (FDA) for VEVYE
(cyclosporine ophthalmic solution) 0.1% to treat the signs and symptoms of dry eye disease.
VEVYE, the development name of which is CyclASol, is a cyclosporine, solubilised solution in a new, water-free excipient.
Recommended Reports
Reports
LOA and PTSR Model - Progesterone in Keratoconjunctivitis Sicca (Dry Eye)
GlobalData
Reports
LOA and PTSR Model - Timbetasin Acetate in Keratoconjunctivitis Sicca (Dry Eye)
GlobalData
View all
It has been designed to address the unmet needs of patients and to provide quick action and well-tolerated dry eye drug therapy.
CyclASol is topical anti-inflammatory and immunomodulating ophthalmic drug solution containing 0.1% cyclosporine A in EyeSol, a unique water-free technology that unlocks the potential of cyclosporine A on the ocular surface.
The water-free formulation of the investigational drug increases residual time on the surface of the eye and improves bioavailability in the target tissues to release the complete potential of cyclosporine A.
Findings from the long-term extension ESSENCE-2 OLE trial showed that CyclASol’s effect was maintained and improved for most endpoints over a treatment period of 52 weeks.
Novaliq medical science and regulatory affairs vice-president Sonja Krösser stated: “We are proud that the FDA approved VEVYE. The safety and efficacy of VEVYE were assessed in a total of 1,369 patients with dry eye disease, of which 738 received VEVYE.
“VEVYE and its novel vehicle were safe, well-tolerated and demonstrated early, consistent and durable therapeutic effects.
“It is an exciting moment when you have followed science that finally led into a new category of water-free ocular therapies addressing unmet medical needs for patients suffering from dry eye disease.”
In May 2023, the company and Bausch + Lomb received
approval from the FDA for MIEBO (perfluorohexyloctane ophthalmic solution)
for the same indication.
Clinical ResultDrug Approval
07 Jun 2023
Aidan McNamee
An illustration of a blood clot. AstraZeneca’s Andexxa works by acting as a decoy for FXa inhibitors, anticoagulants, that would usually bind to the FXa molecule to produce the anticoagulant effect. Image Credit: KATERYNA KON/SCIENCE PHOTO LIBRARY
AstraZeneca
has announced it would stop Andexxa’s Phase IV trial after the study achieved its aims earlier than expected.
Free Report
How is the Biopharmaceutical industry evolving?
2021 was a year of continued innovation and change in the Biopharmaceutical industry. As the COVID-19 pandemic continues to take its toll on businesses worldwide, it’s time to look for new ways to create value, prepare for the future, and remain competitive in the ever-changing landscape.
GlobalData’s expansive report examines the business environment and trends that shape the Biopharmaceutical industry. We highlight the most impactful emerging technologies, as well as the industry, regulatory, and macroeconomic factors that influence growth prospects.
Access the report to:
Benchmark the impact of major themes on the Biopharmaceutical industry.
Gain a deeper "on the ground" perspective through exclusive opinions and analysis from industry respondents.
Evaluate the effects of COVID-19 on the sector.
Download the full report to understand what to expect and how to align your strategies for success.
By GlobalData
Submit
Country Code
UK (+44)
USA (+1)
Algeria (+213)
Andorra (+376)
Angola (+244)
Anguilla (+1264)
Antigua & Barbuda (+1268)
Argentina (+54)
Armenia (+374)
Aruba (+297)
Australia (+61)
Austria (+43)
Azerbaijan (+994)
Bahamas (+1242)
Bahrain (+973)
Bangladesh (+880)
Barbados (+1246)
Belarus (+375)
Belgium (+32)
Belize (+501)
Benin (+229)
Bermuda (+1441)
Bhutan (+975)
Bolivia (+591)
Bosnia Herzegovina (+387)
Botswana (+267)
Brazil (+55)
Brunei (+673)
Bulgaria (+359)
Burkina Faso (+226)
Burundi (+257)
Cambodia (+855)
Cameroon (+237)
Canada (+1)
Cape Verde Islands (+238)
Cayman Islands (+1345)
Central African Republic (+236)
Chile (+56)
China (+86)
Colombia (+57)
Comoros (+269)
Congo (+242)
Cook Islands (+682)
Costa Rica (+506)
Croatia (+385)
Cuba (+53)
Cyprus North (+90392)
Cyprus South (+357)
Czech Republic (+42)
Denmark (+45)
Djibouti (+253)
Dominica (+1809)
Dominican Republic (+1809)
Ecuador (+593)
Egypt (+20)
El Salvador (+503)
Equatorial Guinea (+240)
Eritrea (+291)
Estonia (+372)
Ethiopia (+251)
Falkland Islands (+500)
Faroe Islands (+298)
Fiji (+679)
Finland (+358)
France (+33)
French Guiana (+594)
French Polynesia (+689)
Gabon (+241)
Gambia (+220)
Georgia (+7880)
Germany (+49)
Ghana (+233)
Gibraltar (+350)
Greece (+30)
Greenland (+299)
Grenada (+1473)
Guadeloupe (+590)
Guam (+671)
Guatemala (+502)
Guinea (+224)
Guinea - Bissau (+245)
Guyana (+592)
Haiti (+509)
Honduras (+504)
Hong Kong (+852)
Hungary (+36)
Iceland (+354)
India (+91)
Indonesia (+62)
Iran (+98)
Iraq (+964)
Ireland (+353)
Israel (+972)
Italy (+39)
Jamaica (+1876)
Japan (+81)
Jordan (+962)
Kazakhstan (+7)
Kenya (+254)
Kiribati (+686)
Korea North (+850)
Korea South (+82)
Kuwait (+965)
Kyrgyzstan (+996)
Laos (+856)
Latvia (+371)
Lebanon (+961)
Lesotho (+266)
Liberia (+231)
Libya (+218)
Liechtenstein (+417)
Lithuania (+370)
Luxembourg (+352)
Macao (+853)
Macedonia (+389)
Madagascar (+261)
Malawi (+265)
Malaysia (+60)
Maldives (+960)
Mali (+223)
Malta (+356)
Marshall Islands (+692)
Martinique (+596)
Mauritania (+222)
Mayotte (+269)
Mexico (+52)
Micronesia (+691)
Moldova (+373)
Monaco (+377)
Mongolia (+976)
Montserrat (+1664)
Morocco (+212)
Mozambique (+258)
Myanmar (+95)
Namibia (+264)
Nauru (+674)
Nepal (+977)
Netherlands (+31)
New Caledonia (+687)
New Zealand (+64)
Nicaragua (+505)
Niger (+227)
Nigeria (+234)
Niue (+683)
Norfolk Islands (+672)
Northern Marianas (+670)
Norway (+47)
Oman (+968)
Palau (+680)
Panama (+507)
Papua New Guinea (+675)
Paraguay (+595)
Peru (+51)
Philippines (+63)
Poland (+48)
Portugal (+351)
Puerto Rico (+1787)
Qatar (+974)
Reunion (+262)
Romania (+40)
Russia (+7)
Rwanda (+250)
San Marino (+378)
Sao Tome & Principe (+239)
Saudi Arabia (+966)
Senegal (+221)
Serbia (+381)
Seychelles (+248)
Sierra Leone (+232)
Singapore (+65)
Slovak Republic (+421)
Slovenia (+386)
Solomon Islands (+677)
Somalia (+252)
South Africa (+27)
Spain (+34)
Sri Lanka (+94)
St. Helena (+290)
St. Kitts (+1869)
St. Lucia (+1758)
Sudan (+249)
Suriname (+597)
Swaziland (+268)
Sweden (+46)
Switzerland (+41)
Syria (+963)
Taiwan (+886)
Tajikstan (+7)
Thailand (+66)
Togo (+228)
Tonga (+676)
Trinidad & Tobago (+1868)
Tunisia (+216)
Turkey (+90)
Turkmenistan (+7)
Turkmenistan (+993)
Turks & Caicos Islands (+1649)
Tuvalu (+688)
Uganda (+256)
Ukraine (+380)
United Arab Emirates (+971)
Uruguay (+598)
Uzbekistan (+7)
Vanuatu (+678)
Vatican City (+379)
Venezuela (+58)
Vietnam (+84)
Virgin Islands - British (+1284)
Virgin Islands - US (+1340)
Wallis & Futuna (+681)
Yemen (North)(+969)
Yemen (South)(+967)
Zambia (+260)
Zimbabwe (+263)
Country
UK
USA
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bonaire, Sint Eustatius and Saba
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos Islands
Colombia
Comoros
Congo
Democratic Republic of
the Congo
Cook Islands
Costa Rica
Côte d"Ivoire
Croatia
Cuba
Curaçao
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-Bissau
Guyana
Haiti
Heard Island and McDonald Islands
Holy See
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
North Korea
South Korea
Kuwait
Kyrgyzstan
Lao
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia
Moldova
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Réunion
Romania
Russian Federation
Rwanda
Saint Helena, Ascension and Tristan da Cunha
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan
Tajikistan
Tanzania
Thailand
Timor-Leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
US Minor Outlying Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Vietnam
British Virgin Islands
US Virgin Islands
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Kosovo
By downloading this case study, you acknowledge that GlobalData may share your information with
GlobalData
and that your personal data will be used as described in their
Privacy Policy
Submit
Visit our
Privacy Policy
for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Report.
The ANNEXA-I study (NCT03661528) was designed to assess the use of Andexxa (adexanet alfa) in patients on an oral Factor Xa (FXa) inhibitor treatment including Eliquis (apixaban), which is marketed in the US by
Pfizer
and
Bristol Myers Squibb
, and
Janssen’s
Xarelto (rivaroxaban), who were experiencing an intracranial haemorrhage.
Recommended Reports
Reports
LOA and PTSR Model - Progesterone in Keratoconjunctivitis Sicca (Dry Eye)
GlobalData
Reports
LOA and PTSR Model - Timbetasin Acetate in Keratoconjunctivitis Sicca (Dry Eye)
GlobalData
View all
Andexxa is a modified form of the FXa molecule, an enzyme responsible for blood clotting. The drug works by acting as a decoy for FXa inhibitors, anticoagulants, that would usually bind to the FXa molecule to produce the anticoagulant effect. The treatment had been granted accelerated approval in the US. Under the name Ondexxya, it is also approved in Japan, and conditionally approved in the EU, UK and Switzerland, to treat patients on FXa inhibitors.
The study was stopped after a recommendation from the independent Data and Safety Monitoring Board (DSMB) following a planned interim assessment of efficacy. These early results will be a boon for AstraZeneca, which is currently seeking to broaden its cardiovascular, renal and metabolism offerings, believing it to be a key growth driver for the company.
Millions of people depend on FXa inhibitors to manage potential blood clots; however, they carry a risk of acute major bleeds, necessitating fast acting reversal agents to maintain patient health.
AstraZeneca will now proceed with regulatory filings in the US and EU to convert to full label approval, with full results from the trial to be presented at a forthcoming medical meeting.
Free Report
How is the Biopharmaceutical industry evolving?
2021 was a year of continued innovation and change in the Biopharmaceutical industry. As the COVID-19 pandemic continues to take its toll on businesses worldwide, it’s time to look for new ways to create value, prepare for the future, and remain competitive in the ever-changing landscape.
GlobalData’s expansive report examines the business environment and trends that shape the Biopharmaceutical industry. We highlight the most impactful emerging technologies, as well as the industry, regulatory, and macroeconomic factors that influence growth prospects.
Access the report to:
Benchmark the impact of major themes on the Biopharmaceutical industry.
Gain a deeper "on the ground" perspective through exclusive opinions and analysis from industry respondents.
Evaluate the effects of COVID-19 on the sector.
Download the full report to understand what to expect and how to align your strategies for success.
By GlobalData
Submit
Country Code
UK (+44)
USA (+1)
Algeria (+213)
Andorra (+376)
Angola (+244)
Anguilla (+1264)
Antigua & Barbuda (+1268)
Argentina (+54)
Armenia (+374)
Aruba (+297)
Australia (+61)
Austria (+43)
Azerbaijan (+994)
Bahamas (+1242)
Bahrain (+973)
Bangladesh (+880)
Barbados (+1246)
Belarus (+375)
Belgium (+32)
Belize (+501)
Benin (+229)
Bermuda (+1441)
Bhutan (+975)
Bolivia (+591)
Bosnia Herzegovina (+387)
Botswana (+267)
Brazil (+55)
Brunei (+673)
Bulgaria (+359)
Burkina Faso (+226)
Burundi (+257)
Cambodia (+855)
Cameroon (+237)
Canada (+1)
Cape Verde Islands (+238)
Cayman Islands (+1345)
Central African Republic (+236)
Chile (+56)
China (+86)
Colombia (+57)
Comoros (+269)
Congo (+242)
Cook Islands (+682)
Costa Rica (+506)
Croatia (+385)
Cuba (+53)
Cyprus North (+90392)
Cyprus South (+357)
Czech Republic (+42)
Denmark (+45)
Djibouti (+253)
Dominica (+1809)
Dominican Republic (+1809)
Ecuador (+593)
Egypt (+20)
El Salvador (+503)
Equatorial Guinea (+240)
Eritrea (+291)
Estonia (+372)
Ethiopia (+251)
Falkland Islands (+500)
Faroe Islands (+298)
Fiji (+679)
Finland (+358)
France (+33)
French Guiana (+594)
French Polynesia (+689)
Gabon (+241)
Gambia (+220)
Georgia (+7880)
Germany (+49)
Ghana (+233)
Gibraltar (+350)
Greece (+30)
Greenland (+299)
Grenada (+1473)
Guadeloupe (+590)
Guam (+671)
Guatemala (+502)
Guinea (+224)
Guinea - Bissau (+245)
Guyana (+592)
Haiti (+509)
Honduras (+504)
Hong Kong (+852)
Hungary (+36)
Iceland (+354)
India (+91)
Indonesia (+62)
Iran (+98)
Iraq (+964)
Ireland (+353)
Israel (+972)
Italy (+39)
Jamaica (+1876)
Japan (+81)
Jordan (+962)
Kazakhstan (+7)
Kenya (+254)
Kiribati (+686)
Korea North (+850)
Korea South (+82)
Kuwait (+965)
Kyrgyzstan (+996)
Laos (+856)
Latvia (+371)
Lebanon (+961)
Lesotho (+266)
Liberia (+231)
Libya (+218)
Liechtenstein (+417)
Lithuania (+370)
Luxembourg (+352)
Macao (+853)
Macedonia (+389)
Madagascar (+261)
Malawi (+265)
Malaysia (+60)
Maldives (+960)
Mali (+223)
Malta (+356)
Marshall Islands (+692)
Martinique (+596)
Mauritania (+222)
Mayotte (+269)
Mexico (+52)
Micronesia (+691)
Moldova (+373)
Monaco (+377)
Mongolia (+976)
Montserrat (+1664)
Morocco (+212)
Mozambique (+258)
Myanmar (+95)
Namibia (+264)
Nauru (+674)
Nepal (+977)
Netherlands (+31)
New Caledonia (+687)
New Zealand (+64)
Nicaragua (+505)
Niger (+227)
Nigeria (+234)
Niue (+683)
Norfolk Islands (+672)
Northern Marianas (+670)
Norway (+47)
Oman (+968)
Palau (+680)
Panama (+507)
Papua New Guinea (+675)
Paraguay (+595)
Peru (+51)
Philippines (+63)
Poland (+48)
Portugal (+351)
Puerto Rico (+1787)
Qatar (+974)
Reunion (+262)
Romania (+40)
Russia (+7)
Rwanda (+250)
San Marino (+378)
Sao Tome & Principe (+239)
Saudi Arabia (+966)
Senegal (+221)
Serbia (+381)
Seychelles (+248)
Sierra Leone (+232)
Singapore (+65)
Slovak Republic (+421)
Slovenia (+386)
Solomon Islands (+677)
Somalia (+252)
South Africa (+27)
Spain (+34)
Sri Lanka (+94)
St. Helena (+290)
St. Kitts (+1869)
St. Lucia (+1758)
Sudan (+249)
Suriname (+597)
Swaziland (+268)
Sweden (+46)
Switzerland (+41)
Syria (+963)
Taiwan (+886)
Tajikstan (+7)
Thailand (+66)
Togo (+228)
Tonga (+676)
Trinidad & Tobago (+1868)
Tunisia (+216)
Turkey (+90)
Turkmenistan (+7)
Turkmenistan (+993)
Turks & Caicos Islands (+1649)
Tuvalu (+688)
Uganda (+256)
Ukraine (+380)
United Arab Emirates (+971)
Uruguay (+598)
Uzbekistan (+7)
Vanuatu (+678)
Vatican City (+379)
Venezuela (+58)
Vietnam (+84)
Virgin Islands - British (+1284)
Virgin Islands - US (+1340)
Wallis & Futuna (+681)
Yemen (North)(+969)
Yemen (South)(+967)
Zambia (+260)
Zimbabwe (+263)
Country
UK
USA
Afghanistan
Åland Islands
Albania
Algeria
American Samoa
Andorra
Angola
Anguilla
Antarctica
Antigua and Barbuda
Argentina
Armenia
Aruba
Australia
Austria
Azerbaijan
Bahamas
Bahrain
Bangladesh
Barbados
Belarus
Belgium
Belize
Benin
Bermuda
Bhutan
Bolivia
Bonaire, Sint Eustatius and Saba
Bosnia and Herzegovina
Botswana
Bouvet Island
Brazil
British Indian Ocean Territory
Brunei Darussalam
Bulgaria
Burkina Faso
Burundi
Cambodia
Cameroon
Canada
Cape Verde
Cayman Islands
Central African Republic
Chad
Chile
China
Christmas Island
Cocos Islands
Colombia
Comoros
Congo
Democratic Republic of
the Congo
Cook Islands
Costa Rica
Côte d"Ivoire
Croatia
Cuba
Curaçao
Cyprus
Czech Republic
Denmark
Djibouti
Dominica
Dominican Republic
Ecuador
Egypt
El Salvador
Equatorial Guinea
Eritrea
Estonia
Ethiopia
Falkland Islands
Faroe Islands
Fiji
Finland
France
French Guiana
French Polynesia
French Southern Territories
Gabon
Gambia
Georgia
Germany
Ghana
Gibraltar
Greece
Greenland
Grenada
Guadeloupe
Guam
Guatemala
Guernsey
Guinea
Guinea-Bissau
Guyana
Haiti
Heard Island and McDonald Islands
Holy See
Honduras
Hong Kong
Hungary
Iceland
India
Indonesia
Iran
Iraq
Ireland
Isle of Man
Israel
Italy
Jamaica
Japan
Jersey
Jordan
Kazakhstan
Kenya
Kiribati
North Korea
South Korea
Kuwait
Kyrgyzstan
Lao
Latvia
Lebanon
Lesotho
Liberia
Libyan Arab Jamahiriya
Liechtenstein
Lithuania
Luxembourg
Macao
Macedonia, The Former
Yugoslav Republic of
Madagascar
Malawi
Malaysia
Maldives
Mali
Malta
Marshall Islands
Martinique
Mauritania
Mauritius
Mayotte
Mexico
Micronesia
Moldova
Monaco
Mongolia
Montenegro
Montserrat
Morocco
Mozambique
Myanmar
Namibia
Nauru
Nepal
Netherlands
New Caledonia
New Zealand
Nicaragua
Niger
Nigeria
Niue
Norfolk Island
Northern Mariana Islands
Norway
Oman
Pakistan
Palau
Palestinian Territory
Panama
Papua New Guinea
Paraguay
Peru
Philippines
Pitcairn
Poland
Portugal
Puerto Rico
Qatar
Réunion
Romania
Russian Federation
Rwanda
Saint Helena, Ascension and Tristan da Cunha
Saint Kitts and Nevis
Saint Lucia
Saint Pierre and Miquelon
Saint Vincent and The Grenadines
Samoa
San Marino
Sao Tome and Principe
Saudi Arabia
Senegal
Serbia
Seychelles
Sierra Leone
Singapore
Slovakia
Slovenia
Solomon Islands
Somalia
South Africa
South Georgia and The South
Sandwich Islands
Spain
Sri Lanka
Sudan
Suriname
Svalbard and Jan Mayen
Swaziland
Sweden
Switzerland
Syrian Arab Republic
Taiwan
Tajikistan
Tanzania
Thailand
Timor-Leste
Togo
Tokelau
Tonga
Trinidad and Tobago
Tunisia
Turkey
Turkmenistan
Turks and Caicos Islands
Tuvalu
Uganda
Ukraine
United Arab Emirates
US Minor Outlying Islands
Uruguay
Uzbekistan
Vanuatu
Venezuela
Vietnam
British Virgin Islands
US Virgin Islands
Wallis and Futuna
Western Sahara
Yemen
Zambia
Zimbabwe
Kosovo
By downloading this case study, you acknowledge that GlobalData may share your information with
GlobalData
and that your personal data will be used as described in their
Privacy Policy
Submit
Visit our
Privacy Policy
for more information about our services, how GlobalData may use, process and share your personal data, including information on your rights in respect of your personal data and how you can unsubscribe from future marketing communications. Our services are intended for corporate subscribers and you warrant that the email address submitted is your corporate email address.
Thank you.
Please check your email to download the Report.

Drug ApprovalPhase 2Phase 3Accelerated Approval
100 Deals associated with Thymosin beta-4
Login to view more data
External Link
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | Thymosin beta-4 | - |
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Dry Eye Syndromes | Phase 3 | United States | - | |
| Neurotrophic keratitis | Phase 3 | United States | - | |
| Epidermolysis Bullosa Dystrophica | Preclinical | United States | 22 May 2019 | |
| Epidermolysis Bullosa Dystrophica | Preclinical | United States | 22 May 2019 | |
| Diabetes Mellitus | Preclinical | United States | 01 Dec 2007 | |
| Varicose Ulcer | Preclinical | Poland | 01 Jul 2006 | |
| Varicose Ulcer | Preclinical | Italy | 01 Jul 2006 | |
| Pressure Ulcer | Preclinical | United States | 01 May 2006 | |
| Epidermolysis Bullosa | Preclinical | - | 01 Feb 2006 | |
| Wounds and Injuries | Preclinical | - | 01 Feb 2006 |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 3 | 18 | (RGN-259) | pwgwjdbrlp(bsuyhtvyyj) = menghfhrjd govhkjqfsv (vrsoaszfgx, evsenwtpmq - xsezdqjqui) View more | - | 14 Aug 2023 | ||
Placebo (Placebo) | pwgwjdbrlp(bsuyhtvyyj) = fjmqyvemcx govhkjqfsv (vrsoaszfgx, hpdqplyhun - jqlbgkuanm) View more | ||||||
Phase 3 | 601 | (RGN-259) | nzgnjyzrsw(trfgxhfvrb) = atdkiokwof zwqtfufqdu (twaxwrqrtf, swxbnmjwla - dzhmycghwe) View more | - | 05 Jan 2022 | ||
Placebo (Placebo) | nzgnjyzrsw(trfgxhfvrb) = gpvclxnlvw zwqtfufqdu (twaxwrqrtf, djcwfcnwje - jjiqpbpdtw) View more | ||||||
Phase 3 | Neurotrophic keratitis First line | 18 | (abspsxwprb) = hidcbluagb gpzgtzbhoa (hyjwonfden ) | Positive | 13 Nov 2021 | ||
Placebo | (abspsxwprb) = ycmyelordc gpzgtzbhoa (hyjwonfden ) | ||||||
Phase 3 | 18 | idjqirwvbo(rrjjnlhknp) = aifszathaz iqvrenafkh (kiawperbva ) | Positive | 14 May 2020 | |||
Placebo | idjqirwvbo(rrjjnlhknp) = gxzwodsuwi iqvrenafkh (kiawperbva ) | ||||||
Phase 3 | - | (allhurdmrn) = RGN-259 showed superiority over placebo in reducing corneal fluorescein staining in the change from baseline at days 15 and 29 (p=0.0207 and 0.0254, respectively). niceytcnio (chrcpohyzj ) View more | Positive | 31 Oct 2017 | |||
Placebo | |||||||
NCT01387347 (Pubmed) Manual | Phase 2 | 72 | ijjthqlbsz(wshsoxpcrf) = yxmogwqsiq gamzpwnzff (vfbjsafvkp ) | Positive | 20 May 2015 | ||
Placebo | - | ||||||
Phase 2 | 30 | Thymosin Beta 4+placebo (Placebo) | (bslxvjtyxh) = kmtfhxeplb mpkzleuebq (spzftrsuxo, paotrolink - kaswsfkpjm) View more | - | 10 Jun 2013 | ||
(Thymosin Beta 4) | (bslxvjtyxh) = bpqwcyrbnh mpkzleuebq (spzftrsuxo, ufodnccxwq - qluzoqanwy) View more | ||||||
Phase 2 | 72 | (Thymosin Beta 4) | qzxdbfflfq(qedtlgsdcb) = ocbpnmhjcc lklmcksezb (crhawhwzsg, dnvcptbxep - eyeygtbhkq) View more | - | 24 Dec 2012 | ||
Placebo (Placebo) | qzxdbfflfq(qedtlgsdcb) = kpztjwanzm lklmcksezb (crhawhwzsg, refuzwlnfp - iglmpobstv) View more |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


