Request Demo
Last update 21 Jun 2025
INP-102
Last update 21 Jun 2025
Overview
Basic Info
Drug Type Synthetic peptide |
Synonyms Insulin, POD insulin |
Target |
Action agonists |
Mechanism INSR agonists(Insulin receptor agonists) |
Therapeutic Areas |
Active Indication |
Inactive Indication- |
Originator Organization |
Active Organization |
Inactive Organization- |
License Organization- |
Drug Highest PhasePhase 3 |
First Approval Date- |
Regulation- |
Login to view timeline
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Alzheimer Disease | Phase 3 | - | - |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
Not Applicable | - | - | kymwaafpwj(pkveglwkhb) = ucxjhaybpb tkiuvkojte (ynqluviyll ) View more | - | 14 Jun 2024 | ||
Not Applicable | - | - | (Free Style Libre Pro) | nshfxcvrhw(frlifqphnw) = ctxoenjaip spkvdsmdvr (csrwccrmak, 2.4) | Positive | 14 Jun 2024 | |
(Dexcom reader) | nshfxcvrhw(frlifqphnw) = qibjxtxahe spkvdsmdvr (csrwccrmak, 1.9) | ||||||
Not Applicable | 8 | (Control IQ system (Tandem t:slim insulin pump with Dexcom G6 CGM sensor)) | liomcdpxqh(meyxdvrnrj) = bfrgvlguxl ubsazvhgfz (sakpwnlifb ) View more | Positive | 01 Jun 2024 | ||
Not Applicable | - | - | U-500R insulin | obalvmipos(mnyyelglig) = xdrhmfmpmq wdostfqqfh (hifimujhvl ) View more | - | 05 Oct 2023 | |
Not Applicable | Latent Autoimmune Diabetes in Adults insulin antibodies | GAD-65 | C peptide | - | Plasmapheresis and rituximab | zntymsgayh(qxdixtzbtr) = czomrtipmw tqpaawzryh (drgnbtgndx ) | - | 05 Oct 2023 | |
Not Applicable | - | edaxtwdnkj(sugaiqqvfb) = zqbkatscpa xpodtfhpob (alfjxgdhdx ) View more | Positive | 05 Oct 2023 | |||
Not Applicable | - | - | doirjcutem(fkzfudysay) = A 63-year-old man with diabetes mellitus type 2 presented with an elevated blood glucose of 584mg/dL. He reported 'high' glucose levels, despite adherence to his insulin regimen. He only injected insulin in his abdomen. Over 20 years, his insulin doses increased from Lantus 65 units BID and Humalog 35 units TID to Lantus units 110 BID and Humalog 65 units TID. It was later changed to U-500 at 160 units TID. His Hba1c ranged from 10.7% to 15.4%, most recently 13.6%. Physical examination revealed hyperpigmented and thickened plaques, localized to two large areas lateral to his umbilicus. During his hospital stay, insulin was administered on his arms and thighs. Glucose levels improved on Levemir 55 units daily and Lispro 20 units TID. He was discharged on Insulin U-500 40 units TID with instructions to avoid injecting on his abdomen and to rotate injection sites. dwcrdaledb (snfvkxfsoo ) | - | 01 Nov 2022 | ||
Not Applicable | - | (Children <6 years) | szvxmytglt(wjqrturawo) = srlqthmyds rdrmsznrew (ladotylzlf ) View more | - | 30 Sep 2021 | ||
(Children 6-12 years) | szvxmytglt(wjqrturawo) = mimuozvwmn rdrmsznrew (ladotylzlf ) View more | ||||||
Not Applicable | - | (Omnipod DASH System) | hyozbaoalp(vyvvulutvz) = ssebgvwabi unhxpvbpzs (dzwqyuxnqo, 2.7) View more | - | 30 Sep 2021 | ||
Not Applicable | 49 | tygsasqfpq(rfevyxjqbk) = gaelrarxib ufxwxeviwi (xfljxdxzxd ) | Positive | 29 Sep 2021 |
Login to view more data
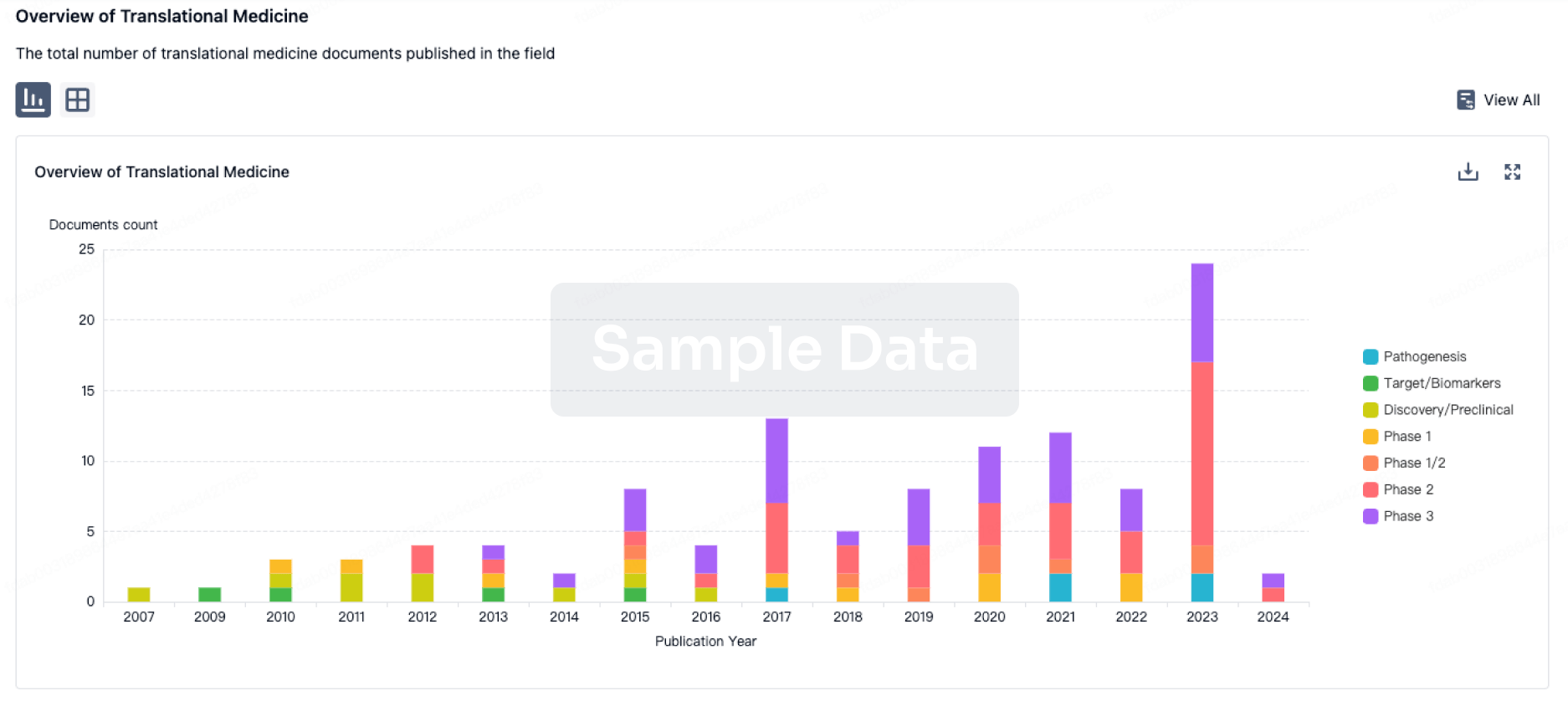
Translational Medicine
Boost your research with our translational medicine data.
login
or
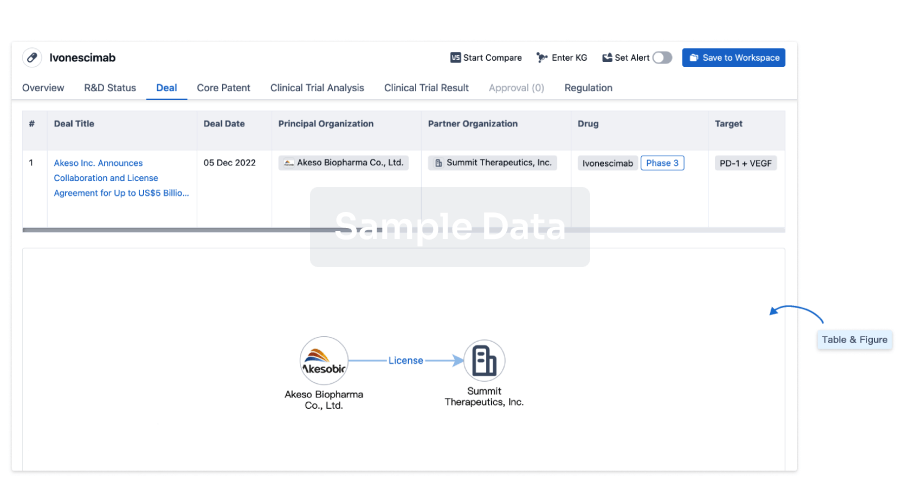
Deal
Boost your decision using our deal data.
login
or
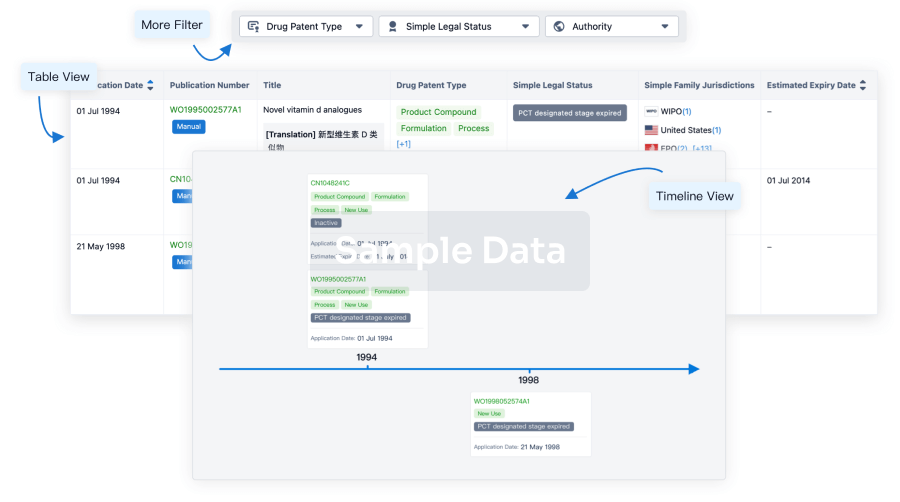
Core Patent
Boost your research with our Core Patent data.
login
or
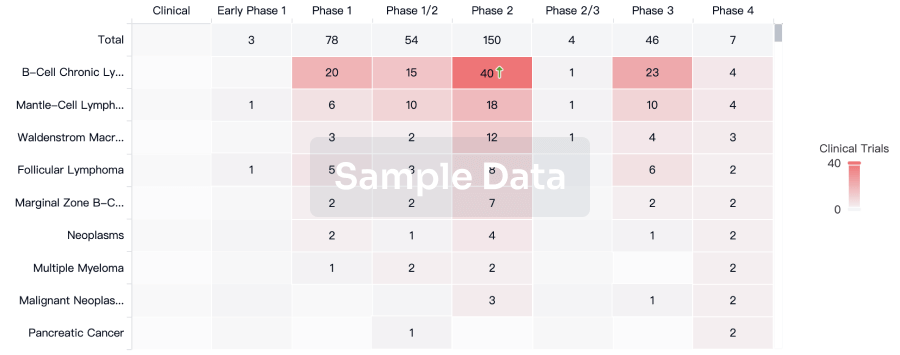
Clinical Trial
Identify the latest clinical trials across global registries.
login
or
Approval
Accelerate your research with the latest regulatory approval information.
login
or

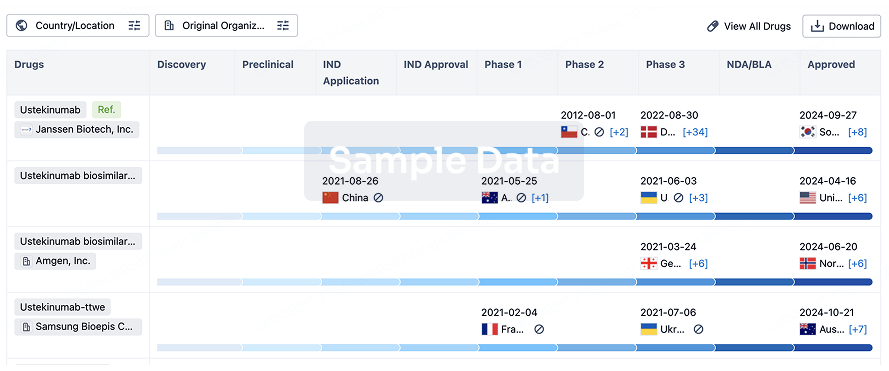
Biosimilar
Competitive landscape of biosimilars in different countries/locations. Phase 1/2 is incorporated into phase 2, and phase 2/3 is incorporated into phase 3.
login
or
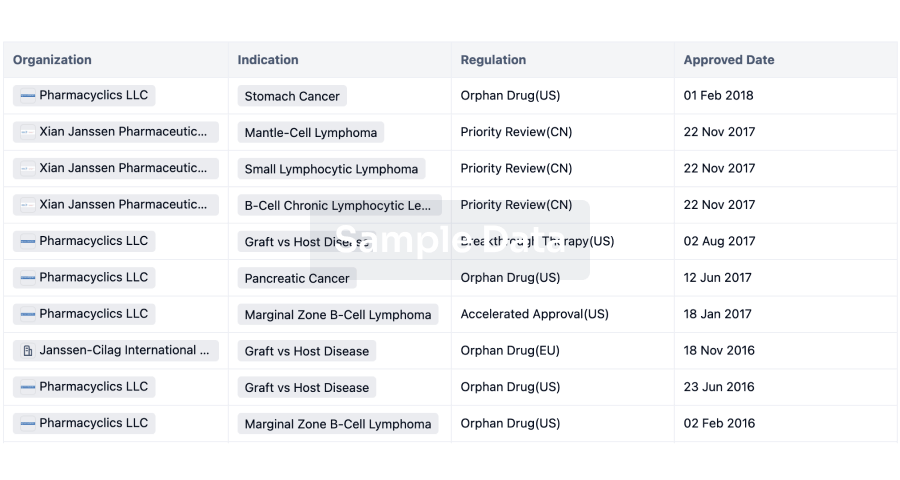
Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or
AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free


