Request Demo
Last update 08 May 2025
ICT-107
Last update 08 May 2025
Overview
Basic Info
Drug Type Therapeutic vaccine |
Synonyms |
Target |
Action modulators |
Mechanism HER2 modulators(Receptor tyrosine-protein kinase erbB-2 modulators), MAGEA1 modulators(Melanoma-associated antigen 1 modulators) |
Therapeutic Areas |
Active Indication- |
Inactive Indication |
Originator Organization |
Active Organization- |
Inactive Organization |
License Organization |
Drug Highest PhaseSuspendedPhase 3 |
First Approval Date- |
Regulation- |
Login to view timeline
Related
2
Clinical Trials associated with ICT-107NCT02546102
A Phase 3 Randomized Double-blind, Controlled Study of ICT-107 With Maintenance Temozolomide (TMZ) in Newly Diagnosed Glioblastoma Following Resection and Concomitant TMZ Chemoradiotherapy
ICT-107 consists of dendritic cells, prepared from autologous mononuclear cells that are pulsed with six synthetic peptides that were derived from tumor associated antigens (TAA) present on glioblastoma tumor cells. This is a Phase 3 study to evaluate ICT-107 in patients with newly diagnosed glioblastoma. Subjects will be randomized to receive standard of care chemoradiation (temozolomide (TMZ) with either ICT-107 or a blinded control. Reinfusion with the pulsed dendritic cells should stimulate cytotoxic T cells to specifically target glioblastoma tumour cells.
Start Date01 Dec 2024 |
Sponsor / Collaborator |
NCT01280552
A Randomized, Double-blind, Controlled Phase IIb Study of the Safety and Efficacy of ICT-107 in Newly Diagnosed Patients With Glioblastoma Multiforme (GBM) Following Resection and Chemoradiation
This is a phase 2, multicenter study to determine the safety and efficacy of ICT-107 in treating a type of brain tumor called Glioblastoma Multiforme (GBM). ICT-107 is an immunotherapy in which the patient's immune response will be stimulated to kill the tumor cells. Patients must be newly diagnosed with GBM and not yet received chemoradiation. Some of the patient's white blood cells (WBC) will be removed and cultured in a laboratory with purified antigens, similar to those on GBM cells. The patient's own WBC/DC that have been exposed to the tumor antigens will then be given back to the patient as a vaccine over several months. The goal is for the ICT-107 vaccine to stimulate the patient's immune response to kill the remaining GBM tumor cells after surgery and chemotherapy.
Start Date01 Jan 2011 |
Sponsor / Collaborator |
100 Clinical Results associated with ICT-107
Login to view more data
100 Translational Medicine associated with ICT-107
Login to view more data
100 Patents (Medical) associated with ICT-107
Login to view more data
5
Literatures (Medical) associated with ICT-10701 Aug 2020·Translational Cancer Research
Vaccines against glioblastoma: reflections on the ICT-107 phase IIb trial
Communications
Author: De Vleeschouwer, Steven
01 Oct 2019·Clinical Cancer ResearchQ1 · MEDICINE
A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma
Q1 · MEDICINE
Article
Author: Glantz, Michael ; Peereboom, David M. ; Pan, Edward ; Curry, William T. ; Wen, Patrick Y. ; Pinilla, Clemencia ; Reardon, David A. ; Santos, Radleigh G. ; Markert, James M. ; Phuphanich, Surasak ; Landolfi, Joseph C. ; Muzikansky, Alona ; Zhu, Jay-Jiguang ; Kim, Lyndon ; Lesser, Glenn J. ; Gruber, Michael ; Kesari, Santosh ; LaRocca, Renato ; Armstrong, Terri S. ; Yu, John S. ; Aiken, Robert D. ; Fink, Karen ; O'Rourke, Donald M.
01 Sep 2019·Cancer Immunology ResearchQ1 · MEDICINE
An Anticancer Drug Cocktail of Three Kinase Inhibitors Improved Response to a Dendritic Cell–Based Cancer Vaccine
Q1 · MEDICINE
Article
Author: Guo, Jitao ; Davila, Eduardo ; Swanson, Steven J. ; Muse, Elena ; Christians, Allison J.
5
News (Medical) associated with ICT-10717 Jun 2022
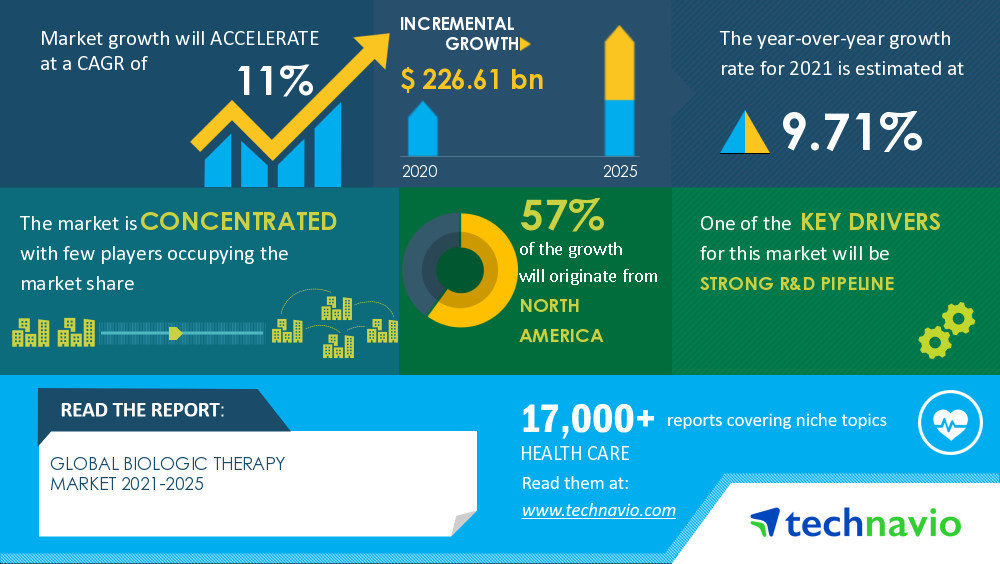
NEW YORK, June 17, 2022 /PRNewswire/ -- One of the key factors driving the
Biologic Therapy Market growth is the
strong R&D pipeline. The efficiency of biologics in the treatment of severe infections, malignancies, and immunological and hormonal disorders is encouraging manufacturers to invest in R&D for the development of biologics.
For instance, monoclonal antibodies constitute one of the fastest-growing segments amongst biological therapies. To date, 88 monoclonal antibodies have been approved for different indications. Some of the examples are ICT-107 for glioblastoma, VGX-3100 for cervical cancer, NeuVax for breast cancer, NexVax2 for celiac disease, CRS-207 for pancreatic cancer, PEV7 for recurrent vulvovaginal candidiasis, and GI-4000 for pancreatic cancer. Similar is the case of biologics meant to be used as vaccines and therapeutic proteins, the pipeline of vendors like AbbVie, Amgen, Johnson & Johnson, and F. Hoffmann-La Roche clearly indicate huge investments in R&D. Apart from manufacturers that are actively involved in research on biologics, many research institutes are also engaged in developing novel biologics through industrial collaborations.
Technavio has announced its latest market research report titled Biologic Therapeutics Market by Product and Geography - Forecast and Analysis 2021-2025
Get more information on Macro & Micro Economic Factor Analysis, Statistical Tools, and Trend Projection at Affordable Pricing options.
Learn More
The Biologic Therapy Market value is set to
grow by USD 226.61 billion, progressing at a CAGR of 11% from 2020 to 2025, as per the latest report by Technavio. Moreover, the market is segmented by
product (antibody therapeutics, vaccines, cell therapy, gene therapy, and other therapies) and geography (North America, Europe, Asia, and ROW).
Biologic Therapy Market 2021-2025: Segmentation
Product
Antibody Therapeutics
Vaccines
Cell Therapy
Gene Therapy
Other Therapies
The biologic therapy market share growth by antibody therapeutics will be significant during the forecast period. Monoclonal antibodies are amongst the most widely sold biologics and are even referred to as the future of medicine.
Monoclonal antibodies are cloned from single-parent cells and are designed to recognize and bind to specific receptors on the surface of the cells. This specificity in action makes monoclonal antibodies more effective than conventional treatments for immunological and chronic ailments like cancer. Owing to these factors, it is expected that the global biologic therapy market will grow during the forecast period.
Geography
North America
Europe
Asia
ROW
57% of the market's growth will originate from North America during the forecast period. The US is the key market for biologic therapy in North America.
Market growth in this region will be slower than the growth of the market in the Asia region. One of the reasons for market growth in the Americas is the increased incidence of chronic diseases.
The rise in the prevalence of major health disorders is mainly due to lifestyle changes and increased consumption of alcohol and tobacco in the US. However, market growth is expected to be only gradual due to the patent expiry of major biologics present in the market.
Biologic Therapy Market 2021-2025: Scope
Biologic Therapy Market size
Biologic Therapy Market trends
Biologic Therapy Market analysis
High cost of biologics is one of the key challenges hindering the biologic therapy market growth. Biologics can be used for the treatment of several types of malignancies, hormonal imbalances, and autoimmune conditions, but they are expensive, which makes them beyond the reach of the economically underprivileged.
The total manufacturing costs, which include the costs related to the clinical trials associated with biologics, account for vendors offering these products at high costs. The development of a biological molecule requires a separate living organism.
Though bacterial production is not that costly, the production of therapeutic agents like monoclonal antibodies and vaccines becomes costly as they involve animal handling. The immunization of the animal and other procedures related to the production of antibodies and vaccines increases the cost of that product. Due to the high price of biologic therapy, many patients are unable to access this effective therapy, and this will remain a major challenge for the market during the forecast period.
Biologic Therapy Market 2021-2025: Vendor Analysis
The report analyzes the market's competitive landscape and offers information on several market vendors, including:
AbbVie Inc.
AstraZeneca Plc
Bristol-Myers Squibb Co.
F. Hoffmann-La Roche Ltd.
GlaxoSmithKline Plc
Johnson and Johnson Inc.
Merck and Co. Inc.
Novartis AG
Pfizer Inc.
Sanofi SA
The biologic therapy market is
concentrated and the vendors are deploying growth strategies such as
partnering with research organizations and hospitals for R&D to compete in the market.
Biologic Therapy Market 2021-2025: Key Highlights
CAGR of the market during the forecast period 2021-2025
Detailed information on factors that will assist biologic therapy market growth during the next five years
Estimation of the biologic therapy market size and its contribution to the parent market
Predictions on upcoming trends and changes in consumer behavior
The growth of the biologic therapy market
Analysis of the market's competitive landscape and detailed information on vendors
Comprehensive details of factors that will challenge the growth of biologic therapy market vendors
Related Reports:
Head and Neck Cancer Diagnostics Market by Diagnostic Methods and Geography - Forecast and Analysis 2020-2024: The head and neck cancer diagnostics market size has the potential to grow by USD 3.41 billion during 2020-2024. The market's growth momentum will accelerate throughout the forecast period.
Latest Exclusive Research Insights Here
Global Spine Biologics Market 2018-2022: The global spine biologics market size will grow by USD 418.72 million during 2018-2022. This industry research report provides a detailed analysis of the market based on product (bone grafts, BMP, BMAC, and others) and geography (the Americas, APAC, and EMEA).
Latest Exclusive Research Insights Here
Table of Contents:
1 Executive Summary
2 Market Landscape
2.1 Market ecosystem
Exhibit 01: Parent market
Exhibit 02: Market characteristics
2.2 Value chain analysis
3 Market Sizing
3.1 Market definition
Exhibit 03: Offerings of vendors included in the market definition
3.2 Market segment analysis
Exhibit 04: Market segments
3.3 Market size 2020
3.4 Market outlook: Forecast for 2020 - 2025
Exhibit 05: Global - Market size and forecast 2020 - 2025 ($ billion)
Exhibit 06: Global market: Year-over-year growth 2020 - 2025 (%)
3.5 Market by application
4 Five Forces Analysis
4.1 Five forces summary
Exhibit 07: Five forces analysis 2020 & 2025
4.2 Bargaining power of buyers
Exhibit 08: Bargaining power of buyers
4.3 Bargaining power of suppliers
Exhibit 09: Bargaining power of suppliers
4.4 Threat of new entrants
Exhibit 10: Threat of new entrants
4.5 Threat of substitutes
Exhibit 11: Threat of substitutes
4.6 Threat of rivalry
Exhibit 12: Threat of rivalry
4.7 Market condition
Exhibit 13: Market condition - Five forces 2020
5 Market Segmentation by Product
5.1 Market segments
Exhibit 14: Product - Market share 2020-2025 (%)
5.2 Comparison by Product
Exhibit 15: Comparison by Product
5.3 Antibody therapeutics - Market size and forecast 2020-2025
Exhibit 16: Antibody therapeutics - Market size and forecast 2020-2025 ($ billion)
Exhibit 17: Sales of major monoclonal antibodies 2018-2020 ($ million)
Exhibit 18: Antibody therapeutics - Year-over-year growth 2020-2025 (%)
5.4 Vaccines - Market size and forecast 2020-2025
Exhibit 19: Vaccines - Market size and forecast 2020-2025 ($ billion)
Exhibit 20: Vendors offering vaccines
Exhibit 21: Vaccines - Year-over-year growth 2020-2025 (%)
5.5 Cell therapy - Market size and forecast 2020-2025
Exhibit 22: Cell therapy - Market size and forecast 2020-2025 ($ billion)
Exhibit 23: Cell therapy - Year-over-year growth 2020-2025 (%)
5.6 Gene therapy - Market size and forecast 2020-2025
Exhibit 24: Gene therapy - Market size and forecast 2020-2025 ($ billion)
Exhibit 25: Gene therapy - Year-over-year growth 2020-2025 (%)
5.7 Other therapies - Market size and forecast 2020-2025
Exhibit 26: Other therapies - Market size and forecast 2020-2025 ($ billion)
Exhibit 27: Other therapies - Year-over-year growth 2020-2025 (%)
5.8 Market opportunity by Product
Exhibit 28: Market opportunity by Product
6 Customer landscape
7 Geographic Landscape
7.1 Geographic segmentation
Exhibit 30: Market share by geography 2020-2025 (%)
7.2 Geographic comparison
Exhibit 31: Geographic comparison
7.3 North America - Market size and forecast 2020-2025
Exhibit 32: North America - Market size and forecast 2020-2025 ($ billion)
Exhibit 33: North America - Year-over-year growth 2020-2025 (%)
7.4 Europe - Market size and forecast 2020-2025
Exhibit 34: Europe - Market size and forecast 2020-2025 ($ billion)
Exhibit 35: Europe - Year-over-year growth 2020-2025 (%)
7.5 Asia - Market size and forecast 2020-2025
Exhibit 36: Asia - Market size and forecast 2020-2025 ($ billion)
Exhibit 37: Asia - Year-over-year growth 2020-2025 (%)
7.6 ROW - Market size and forecast 2020-2025
Exhibit 38: ROW - Market size and forecast 2020-2025 ($ billion)
Exhibit 39: ROW - Year-over-year growth 2020-2025 (%)
7.7 Key leading countries
Exhibit 40: Key leading countries
7.8 Market opportunity by geography
Exhibit 41: Market opportunity by geography ($ billion)
8 Drivers, Challenges, and Trends
8.1 Market drivers
8.2 Market challenges
Exhibit 43: Impact of drivers and challenges
8.3 Market trends
9 Vendor Landscape
9.1 Overview
Exhibit 44: Vendor landscape
9.2 Landscape disruption
Exhibit 45: Landscape disruption
Exhibit 46: Industry risks
10 Vendor Analysis
10.1 Vendors covered
Exhibit 47: Vendors covered
10.2 Market positioning of vendors
Exhibit 48: Market positioning of vendors
10.3 AbbVie Inc.
Exhibit 49: AbbVie Inc. - Overview
Exhibit 50: AbbVie Inc. - Product and service
Exhibit 51: AbbVie Inc. – Key news
Exhibit 52: AbbVie Inc. - Key offerings
10.4 AstraZeneca Plc
Exhibit 53: AstraZeneca Plc - Overview
Exhibit 54: AstraZeneca Plc - Product and service
Exhibit 55: AstraZeneca Plc – Key news
Exhibit 56: AstraZeneca Plc - Key offerings
10.5 Bristol-Myers Squibb Co.
Exhibit 57: Bristol-Myers Squibb Co. - Overview
Exhibit 58: Bristol-Myers Squibb Co. - Product and service
Exhibit 59: Bristol-Myers Squibb Co. – Key news
Exhibit 60: Bristol-Myers Squibb Co. - Key offerings
10.6 F. Hoffmann-La Roche Ltd.
Exhibit 61: F. Hoffmann-La Roche Ltd. - Overview
Exhibit 62: F. Hoffmann-La Roche Ltd. - Business segments
Exhibit 63: F. Hoffmann-La Roche Ltd. – Key news
Exhibit 64: F. Hoffmann-La Roche Ltd. - Key offerings
Exhibit 65: F. Hoffmann-La Roche Ltd. - Segment focus
10.7 GlaxoSmithKline Plc
Exhibit 66: GlaxoSmithKline Plc - Overview
Exhibit 67: GlaxoSmithKline Plc - Business segments
Exhibit 68: GlaxoSmithKline Plc – Key news
Exhibit 69: GlaxoSmithKline Plc - Key offerings
Exhibit 70: GlaxoSmithKline Plc - Segment focus
10.8 Johnson and Johnson Inc.
Exhibit 71: Johnson and Johnson Inc. - Overview
Exhibit 72: Johnson and Johnson Inc. - Business segments
Exhibit 73: Johnson and Johnson Inc. – Key news
Exhibit 74: Johnson and Johnson Inc. - Key offerings
Exhibit 75: Johnson and Johnson Inc. - Segment focus
10.9 Merck and Co. Inc.
Exhibit 76: Merck and Co. Inc. - Overview
Exhibit 77: Merck and Co. Inc. - Business segments
Exhibit 78: Merck and Co. Inc. – Key news
Exhibit 79: Merck and Co. Inc. - Key offerings
Exhibit 80: Merck and Co. Inc. - Segment focus
10.10 Novartis AG
Exhibit 81: Novartis AG - Overview
Exhibit 82: Novartis AG - Business segments
Exhibit 83: Novartis AG – Key news
Exhibit 84: Novartis AG - Key offerings
Exhibit 85: Novartis AG - Segment focus
10.11 Pfizer Inc.
Exhibit 86: Pfizer Inc. - Overview
Exhibit 87: Pfizer Inc. - Business segments
Exhibit 88: Pfizer Inc. – Key news
Exhibit 89: Pfizer Inc. - Key offerings
10.12 Sanofi SA
Exhibit 90: Sanofi SA - Overview
Exhibit 91: Sanofi SA - Business segments
Exhibit 92: Sanofi SA – Key news
Exhibit 93: Sanofi SA - Key offerings
Exhibit 94: Sanofi SA - Segment focus
11 Appendix
11.1 Scope of the report
11.2 Currency conversion rates for US$
Exhibit 95: Currency conversion rates for US$
11.3 Research methodology
Exhibit 96: Research Methodology
Exhibit 97: Validation techniques employed for market sizing
Exhibit 98: Information sources
11.4 List of abbreviations
Exhibit 99: List of abbreviations
About Us
Technavio is a leading global technology research and advisory company. Their research and analysis focus on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contact
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email: [email protected]
Website:
SOURCE Technavio
VaccineGene TherapyAntibodyCell TherapyCollaborate
07 Mar 2016
March 7, 2016
By
Mark Terry
, BioSpace.com Breaking News Staff
Hampton, N.J.-based
Celldex Therapeutics
announced
today that its Rintega cancer vaccine had failed to meet its primary endpoint and the study was being terminated. The company’s
shares plunged
by more than half in premarket trading on the news.
An independent
Data Safety and Monitoring Board (DSMB)
determined that the study should be halted based on interim analysis of data from the Phase III, ACT IV study. Rintega (rindopepimut) is a cancer vaccine designed to focus the immune system on a specific tumor type, in this case, in patients with newly diagnosed EGFRviii-positive glioblastoma (GMB), a type of brain cancer.
GMB is the most prevalent and aggressive form of brain cancer, and according to the
National Cancer Institute
about 22,850 new cases were diagnosed in the U.S. in 2015. There were an estimated 15,320 related deaths. Fewer than 10 percent of patients who have glioblastoma recurrence live more than five years.
The
DSMB’s analysis
found that Rintega cut the risk of death by only 1 percent compared to the control arm of the study. But the median range was worse—patients on Rintega only survived 20.4 months compared to 21.1 months in the control arm.
“We are extremely disappointed for patients that the ACT IV study was not successful,” said
Anthony Marucci
, Celldex co-founder, president and chief executive officer, in a statement. “On behalf of Celldex, I want to express our gratitude to the ACT IV investigators, patients and families who participated in this trial. While this is certainly not the desired outcome, we remain steadfast believers in the power of immunotherapy to transform the future of cancer treatments.”
Patients who were receiving Rintega will be offered the drug on a compassionate use basis.
Celldex’s have had a rough year in general. Shares traded a year ago, on March 19, 2015, for $31.78. On Aug. 21, they traded for $13.53, then dropped to $10.38 on Sept. 29. Shares rebounded a bit on Nov. 23 to $18.11, then dropped on Feb. 10, 2016 to $6.45. Shares are currently trading for $8.19.
Celldex has a fairly
vigorous pipeline
. Its glembatumumab vedotin is an antibody drug conjugate being studied in a trial on triple-negative breast cancer patients. Enrollment for patients is expected to be completed in the second half of this year. The company’s varlilumab, a monoclonal antibody that targets CD27, is being investigated in early clinical trials in solid tumors, some in conjunction with Bristol-Myers Squibb’s checkpoint inhibitor, Opdivo.
Although the idea of a cancer vaccine is intriguing, there hasn’t been many successes to date. ImmunoCellular Therapeutics’s GBM vaccine ICT-107 failed trials in 2013. A GBM vaccine called DCVax, developed by
Northwest Biotherapeutics
, was halted in August for unexplained reasons.
So far Provenge, a vaccine for prostate cancer, is the only approved cancer vaccine, although Dendreon declared bankruptcy and was
acquired
by
Valeant Pharmaceuticals
. Provenge was considered a very promising drug, but its price tag was $93,000 for a course of treatment. Physicians didn’t pick it up and insurers were reluctant to pay for it.
“
Dendreon
combined undue optimism in sales projections with excessive risk in its capital structure,” said
Erik Gordon
, professor at the
University of Michigan
’s Ross School of Business in a statement from Feb. 2015. “It is a case of moderate product success and immoderate management failure.”
Although not always cited as a cancer vaccine, the vaccines against human papilloma virus (HPV) are actually vaccines against certain types of cancer, including cervical cancer, anal cancer, vaginal cancer, vulvar cancer and some types of mouth cancer. Gardisil is marketed by
Merck
, and Cervarix is marketed by
GlaxoSmithKline
.
VaccineAcquisitionPhase 3ImmunotherapymRNA
30 Apr 2015
LOS ANGELES, April 30, 2015 /PRNewswire/ -- ImmunoCellular Therapeutics, Ltd. ("ImmunoCellular") (NYSE MKT: IMUC) today announced that it plans to report financial results for the first quarter 2015 on Monday, May 11, 2015. The Company also plans to hold a conference call and webcast on Tuesday, May 12, 2015, at 5:00 pm ET to discuss the first quarter 2015 financial results and provide a business update. The call will be hosted by Andrew Gengos, President and CEO.
LIVE CALL: (877) 853-5636 (toll-free); international dial-in: (631) 291-4544; conference code 30452546.
WEBCAST: Interested parties who wish to listen to the webcast should visit the Investor Relations section of ImmunoCellular's website at , under the Events and Presentations tab. A replay of the webcast will be available one hour after the conclusion of the event.
The conference call will contain forward-looking statements. The information provided on the teleconference is accurate only at the time of the conference call, and ImmunoCellular will take no responsibility for providing updated information except as required by law.
About ImmunoCellular Therapeutics, Ltd.
ImmunoCellular Therapeutics, Ltd. is a Los Angeles-based clinical-stage company that is developing immune-based therapies for the treatment of brain and other cancers. ImmunoCellular has concluded a phase II trial of its lead product candidate, ICT-107, a dendritic cell-based vaccine targeting multiple tumor-associated antigens for glioblastoma. ImmunoCellular's pipeline also includes: ICT-121, a dendritic cell vaccine targeting CD133; ICT-140, a dendritic cell vaccine targeting ovarian cancer antigens and cancer stem cells; and the Stem-to-T-cell research program which engineers the patient's hematopoietic stem cells to generate antigen-specific cancer killing T-cells. To learn more about ImmunoCellular, please visit .
Contact:
ImmunoCellular Therapeutics, Ltd.
Investor Relations
Jane Green
415.348.0010 direct
415.652.4819 mobile
jane@jmgcomm.com
To view the original version on PR Newswire, visit:
SOURCE ImmunoCellular Therapeutics, Ltd.
Help employers find you! Check out all the jobs and post your resume.
VaccineFinancial Statement
100 Deals associated with ICT-107
Login to view more data
R&D Status
10 top R&D records. to view more data
Login
| Indication | Highest Phase | Country/Location | Organization | Date |
|---|---|---|---|---|
| Glioblastoma Multiforme | Phase 3 | Canada | 01 Dec 2024 | |
| Glioblastoma Multiforme | Phase 3 | Austria | 01 Dec 2024 | |
| Glioblastoma Multiforme | Phase 3 | United States | 01 Dec 2024 | |
| Glioblastoma | Phase 3 | Spain | - | |
| Glioblastoma | Phase 3 | Netherlands | - | |
| Glioblastoma | Phase 3 | Austria | - | |
| Glioblastoma | Phase 3 | Canada | - | |
| Glioblastoma | Phase 3 | United States | - | |
| Glioblastoma | Phase 3 | Germany | - |
Login to view more data
Clinical Result
Clinical Result
Indication
Phase
Evaluation
View All Results
| Study | Phase | Population | Analyzed Enrollment | Group | Results | Evaluation | Publication Date |
|---|
Phase 2 | Glioblastoma HLA-A02 Positive | 124 | six synthetic peptide+ICT-107 | (cabpktsjcy) = cizxuupqxm ogxlltqxmt (cowagqchkg ) View more | Positive | 01 Oct 2019 | |
six synthetic peptide | - | ||||||
Phase 2 | 124 | (unslugqtyk) = zzjwxkdjeb bhxejebfzj (afyypqzayi ) View more | Positive | 20 May 2014 | |||
(unslugqtyk) = giqijmnoda bhxejebfzj (afyypqzayi ) | |||||||
Phase 1 | 16 | (vbucsavbnf) = iesyfdxzbf xcqgbvaoax (zkrnyryero ) | - | 20 May 2011 |
Login to view more data
Translational Medicine
Boost your research with our translational medicine data.
login
or

Deal
Boost your decision using our deal data.
login
or

Core Patent
Boost your research with our Core Patent data.
login
or

Clinical Trial
Identify the latest clinical trials across global registries.
login
or

Approval
Accelerate your research with the latest regulatory approval information.
login
or

Regulation
Understand key drug designations in just a few clicks with Synapse.
login
or

AI Agents Built for Biopharma Breakthroughs
Accelerate discovery. Empower decisions. Transform outcomes.
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Empower better decisions with the latest in pharmaceutical intelligence.
Bio
Bio Sequences Search & Analysis
Sign up for free
Chemical
Chemical Structures Search & Analysis
Sign up for free
