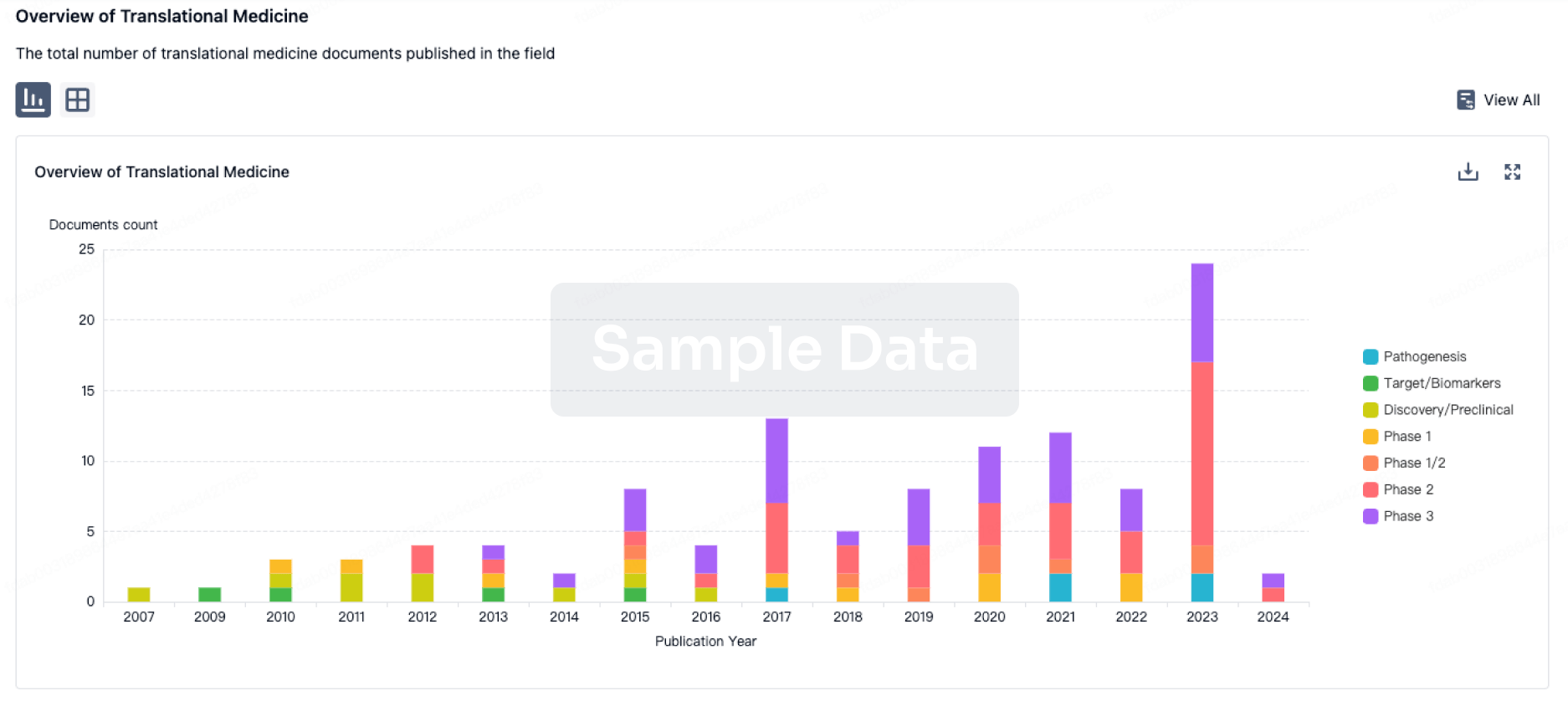
The MASH treatment landscape is evolving rapidly, driven by a diverse array of therapeutic classes, each with unique mechanisms and potential impacts. The forecast period reveals significant shifts and emerging trends that will shape future market dynamics.
LAS VEGAS, Jan. 30, 2025 /PRNewswire/ -- DelveInsight's
Metabolic Dysfunction-Associated Steatohepatitis Market Insights report includes a comprehensive understanding of current treatment practices, MASH emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan].
Key Takeaways from the Metabolic Dysfunction-Associated Steatohepatitis Market Report
According to DelveInsight's analysis, the market size for MASH was found to be
USD 2.1 billion in the 7MM in 2023.
In 2023, there were an estimated
42 million prevalent cases of MASH in the 7MM. Out of these, a total of
~15 million cases were diagnosed, and this number is projected to increase by the end of 2034 in the 7MM.
Leading MASH companies such as
Inventiva Pharma, Novo Nordisk A/S, Cirius Therapeutics, Akero Therapeutics, 89bio, Boehringer Ingelheim, Zealand Pharma, Galectin Therapeutics, Lipocine, Viking Therapeutics, Eli Lilly and Company, Boston Pharmaceuticals, Pfizer, HighTide Biopharma, CytoDyn, Merck & Co., Hanmi Pharmaceutical, Hepagene (Shanghai), Hepion Pharmaceuticals, Enyo Pharmaceuticals, Gilead Sciences, Poxel SA, Zydus Therapeutics, Sagimet Biosciences, Ionis Pharmaceuticals, Corcept Therapeutics, and others are developing novel MASH drugs that can be available in the MASH market in the coming years.
The promising MASH therapies in the pipeline include
Lanifibranor (IVA337), Semaglutide, Azemiglitazone (MSDC-0602K), Efruxifermin (EFX), BIO89-100 (Pegozafermin), Survodutide (BI 456906), GR-MD-02 (Belapectin), LPCN1144, VK2809, Tirzepatide, BOS-580, Ervogastat (PF-06865571) + Clesacostat (PF-05221304), HTD1801, Leronlimab (PRO 140), Efinopegdutide, HPG1860, Rencofilstat (CRV431), EYP001 (Vonafexor), Semaglutide/Cilofexor/Firsocostat, PXL065, Saroglitazar Magnesium, Denifanstat (TVB-2640), ION224, Miricorilant (CORT118335), and others.
In
March 2024, Madrigal Pharmaceuticals' groundbreaking product, REZDIFFRA (resmetirom), a once-daily, oral THR-ß agonist, received accelerated endorsement from the US FDA based on results from the Phase III MAESTRO-NASH trial. This approval marks a significant stride in the medical landscape, as REZDIFFRA becomes the inaugural and sole FDA-sanctioned therapy for adults afflicted with non-cirrhotic MASH, accompanied by moderate to advanced liver scarring (fibrosis) corresponding to stages F2–F3 fibrosis.
Discover which therapies are expected to grab the major MASH market share @
Metabolic Dysfunction-Associated Steatohepatitis Market Report
Metabolic Dysfunction-Associated Steatohepatitis Overview
Metabolic dysfunction-associated steatohepatitis (MASH) is a progressive liver disease that stems from metabolic dysfunction, often linked to obesity, diabetes, and other conditions of metabolic syndrome. MASH is characterized by the accumulation of fat in liver cells, accompanied by inflammation and liver cell injury, which can progress to fibrosis, cirrhosis, or even liver cancer.
The primary drivers of MASH include insulin resistance, obesity, type 2 diabetes, and dyslipidemia. Genetic predisposition and a sedentary lifestyle also play significant roles. Environmental factors, such as a poor diet high in sugars and fats, exacerbate the condition.
MASH is often asymptomatic in its early stages. When symptoms occur, they can include fatigue, vague abdominal discomfort, or pain in the upper right quadrant. In advanced stages, signs of liver dysfunction such as jaundice, swelling of the abdomen or legs, and confusion may arise.
Diagnosis involves a combination of clinical history, physical examination, and diagnostic tests. Blood tests measuring liver enzymes (ALT, AST) often indicate liver inflammation. Imaging techniques like ultrasound, MRI, or FibroScan can identify liver fat and fibrosis. In some cases, a liver biopsy is required to confirm the diagnosis and assess disease severity.
Metabolic Dysfunction-Associated Steatohepatitis Epidemiology Segmentation
The MASH epidemiology section provides insights into the historical and current MASH patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The MASH market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
Prevalent Cases of MASH
Diagnosed Prevalent Cases of MASH
Gender-specific Diagnosed Prevalent Cases of MASH
Severity-specific Diagnosed Prevalent Cases of MASH
Metabolic Dysfunction-Associated Steatohepatitis Treatment Market
The approval of
REZDIFFRA (resmetirom) in March 2024 represents a pivotal achievement in medical innovation, transforming the treatment landscape for MASH disease. This groundbreaking therapy addresses the root causes of MASH, offering renewed hope to patients grappling with this challenging condition. Clinical trials have shown impressive results, with REZDIFFRA effectively reducing symptoms like inflammation and fibrosis, enhancing liver function, and improving patients' quality of life. By providing healthcare professionals with a robust treatment option, this approval addresses a critical unmet need and has the potential to significantly alleviate the complications linked to advanced liver disease.
The prevalence of MASLD is strongly linked to type 2 diabetes mellitus and obesity, particularly in individuals with a higher body mass index. However, MASLD occurrence is reduced in T2DM patients receiving treatments such as sodium-glucose cotransporter-2 (SGLT2) inhibitors, GLP-1 receptor agonists, and insulin. Vitamin E, with its antioxidant properties, is regarded as a first-line pharmacological option for managing MASH, especially when dietary and lifestyle interventions are insufficient.
Antifibrotic agents can help prevent the progression of liver fibrosis and MASLD into fibrotic MASH. The role of pioglitazone, an anti-diabetic medication, in improving MASH histology in T2DM patients is well-documented, but concerns remain regarding potential side effects like weight gain, fluid retention, cancer risk, and bone fractures. Additional therapeutic targets for MASLD and MASH include G protein-coupled receptors (GPCRs), estrogen-related receptor alpha (ERRα), bone morphogenetic proteins (BMPs), and KLFs. Bariatric surgery, or weight loss surgery, is the most effective intervention for treating obesity and diabetes, as it reduces food absorption while influencing gut hormone secretion and metabolic function.
To know more about MASH treatment guidelines, visit @
Metabolic Dysfunction-Associated Steatohepatitis Management
Metabolic Dysfunction-Associated Steatohepatitis Pipeline Therapies and Key Companies
Lanifibranor (IVA337): Inventiva Pharma
Semaglutide: Novo Nordisk A/S
Azemiglitazone (MSDC-0602K): Cirius Therapeutics
Efruxifermin (EFX): Akero Therapeutics
BIO89-100 (Pegozafermin): 89bio
Survodutide (BI 456906): Boehringer Ingelheim/Zealand Pharma
GR-MD-02 (Belapectin): Galectin Therapeutics
LPCN1144: Lipocine
VK2809: Viking Therapeutics
Tirzepatide: Eli Lilly and Company
BOS-580: Boston Pharmaceuticals
Ervogastat (PF-06865571) + Clesacostat (PF-05221304): Pfizer
HTD1801: HighTide Biopharma
Leronlimab (PRO 140): CytoDyn
Efinopegdutide: Merck & Co./Hanmi Pharmaceutical
HPG1860: Hepagene (Shanghai)
Rencofilstat (CRV431): Hepion Pharmaceuticals
EYP001 (Vonafexor): Enyo Pharmaceuticals
Semaglutide/ Cilofexor/ Firsocostat: Gilead Sciences
PXL065: Poxel SA
Saroglitazar Magnesium: Zydus Therapeutics
Denifanstat (TVB-2640): Sagimet Biosciences
ION224: Ionis Pharmaceuticals
Miricorilant (CORT118335): Corcept Therapeutics
Discover more about MASH drugs in development @
Metabolic Dysfunction-Associated Steatohepatitis Clinical Trials
Metabolic Dysfunction-Associated Steatohepatitis Market Dynamics
The MASH market dynamics are expected to change in the coming years.
Growing research activities and multiple clinical trials for MASH, driven by the
rapid surge in its prevalence due to rising obesity and type 2 diabetes rates, highlight an active
drug development pipeline and an expanding market size. The large pool of patients and lucrative growth opportunities present attractive prospects for key players, further supported by
ongoing preclinical studies aimed at advancing imaging techniques for MASH diagnosis, potentially eliminating the need for invasive biopsy-based histopathological confirmation.
Furthermore, potential therapies are being investigated for the treatment of MASH, and it is safe to predict that the treatment space will significantly impact the MASH market during the forecast period. Moreover, the
anticipated introduction of emerging therapies with improved efficacy and a further
improvement in the diagnosis rate are expected to drive the growth of the MASH market in the 7MM.
However, several factors may impede the growth of the MASH market.
Lack of awareness and negligence in the early stages of MASH by physicians often lead to disease progression, culminating in irreversible damage where liver transplantation becomes the only viable option. Diagnosing advanced MASH typically requires procedures like liver biopsy, which are
costly, invasive, and risky.
Regulatory challenges also pose hurdles, as the FDA mandates achieving one MASH endpoint for approval, while the EMA's draft guidance requires efficacy in both endpoints, potentially delaying first-mover approvals in major European markets. Additionally,
access to expensive MASH treatments may be limited in certain regions, further hindering patient adoption.
Moreover, MASH treatment poses a
significant economic burden and disrupts patients' overall well-being and QOL. Furthermore, MASH market growth may be offset by
failures and discontinuation of emerging therapies,
unaffordable pricing,
market access and reimbursement issues, and a
shortage of healthcare specialists. In addition, the
undiagnosed, unreported cases and the
unawareness about the disease may also impact MASH market growth.
Scope of the
Metabolic Dysfunction-Associated Steatohepatitis
Market Report
Therapeutic Assessment: Metabolic Dysfunction-Associated Steatohepatitis current marketed and emerging therapies
Metabolic Dysfunction-Associated Steatohepatitis
Market Dynamics: Key Market Forecast Assumptions of Emerging Metabolic Dysfunction-Associated Steatohepatitis Drugs and Market Outlook
Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
Unmet Needs, KOL's views, Analyst's views, Metabolic Dysfunction-Associated Steatohepatitis Market Access and Reimbursement
Download the report to understand which factors are driving MASH market trends @
Metabolic Dysfunction-Associated Steatohepatitis Market Trends
Table of Contents
Related Reports
Non-Alcoholic Steatohepatitis Epidemiology Forecast
Non-Alcoholic Steatohepatitis Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted NASH epidemiology in the 7MM, i.e., the United States, EU4 (Germany, France, Italy, Spain), the United Kingdom, and Japan.
Nonalcoholic Steatohepatitis Pipeline
Nonalcoholic Steatohepatitis Pipeline Insight
– 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key nonalcoholic steatohepatitis companies, including
Madrigal Pharmaceuticals, Intercept Pharmaceuticals, Cirius Therapeutics, Novo Nordisk, Inventiva, Galmed Pharmaceuticals, AstraZeneca, Galectin Therapeutics, Viking Therapeutics, Eli Lilly and Company, Terns Pharmaceuticals, Sinew Pharma, 89bio, Inc., Eccogene, Novartis Pharmaceuticals, Poxel SA, AngioLab, Pfizer, Arrowhead Pharma, LG Chem, Redx Pharma, Lipocine, Inc., CytoDyn, Inc., Alnylam Pharmaceuticals, Inc., Chemomab Therapeutics, NuSirt Biopharma, HK inno. N, Aligos Therapeutics, Altimmune, Inc., Kowa Pharmaceutical, Ionis Pharmaceuticals, NorthSea Therapeutics, Rivus Pharmaceuticals, Hanmi Pharmaceutical, Hepagene Therapeutics, HighTide Biopharma, Akero Therapeutics, Merck Sharp & Dohme LLC, Cascade Pharmaceuticals, Hepion Pharmaceuticals, Chipscreen Biosciences, Boston Pharmaceuticals, Bristol-Myers Squibb, Sunshine Lake Pharma, GSK plc., Future Medicine, Gilead Sciences, ENYO Pharma, Histogen, among others.
Non-Alcoholic Fatty Liver Disease Market
Non-Alcoholic Fatty Liver Disease
Market Insight, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key NAFLD companies, including
MediciNova, Eli Lilly and Company, AstraZeneca, Inventiva Pharma, Oasis Pharmaceuticals, LLC, Madrigal Pharmaceuticals, Inc., BioMarin Pharmaceutical, GlaxoSmithKline, Zydus Therapeutics Inc., Akero Therapeutics, Inc, Pfizer, Boehringer Ingelheim, Neuraly, Inc., Merck Sharp & Dohme LLC, Rivus Pharmaceuticals, Inc., Hepion Pharmaceuticals, Inc.
, among others.
Non-Alcoholic Fatty Liver Disease Pipeline
Non-Alcoholic Fatty Liver Disease Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key NAFLD companies, including
BeiGene, Inventiva Pharma, Cirius Therapeutics, Madrigal Pharma, Novo Nordisk, Galmed Pharmaceuticals, AstraZeneca, Galectin Therapeutics, Viking Therapeutics, Dr. Falk Pharma GmbH, Sagimet Biosciences, Eli Lilly and Company, Terns Pharmaceuticals, Sinew Pharma, Novartis Pharmaceuticals, Afimmune, Poxel SA, AngioLab, Pfizer, Oramed Pharmaceuticals, Can Fite Biopharma, MediciNova, Metacrine, Inc., Lipocine, Inc., CytoDyn, Inc., Alnylam Pharmaceuticals, Inc., Mitsubishi Tanabe Pharma, Chemomab Therapeutics, NuSirt Biopharma, HK inno.N, Kowa Pharmaceutical, Ionis Pharmaceuticals, NorthSea Therapeutics, Rivus Pharmaceuticals, Hanmi Pharmaceutical, Hepagene Therapeutics, HighTide Biopharma, Akero Therapeutics, Enanta Pharmaceuticals, Cascade Pharmaceuticals, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Contact Us
Shruti Thakur
[email protected]
+14699457679
Logo:
SOURCE DelveInsight Business Research, LLP
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED