Ikena further sheds staff and pipeline in bid to maximise value
Phase 1Drug ApprovalClinical ResultFinancial StatementPhase 2
Preview
Source: Pharmaceutical Technology
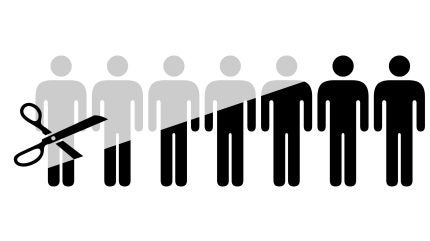
Ikena will terminate approximately 18 employees, 53% of its workforce, by the end of Q3 this year. Image Credit: Zern Liew / Shutterstock.
For the second time this year, US-based Ikena Oncology has announced plans for workforce reduction and pipeline prioritisation.
Recommended Buyer's Guides

Preview
Source: Pharmaceutical Technology
Buyer's Guide
Top Guide for Drug Delivery Systems

Preview
Source: Pharmaceutical Technology
Buyer's Guide
Top Guide for Cleanrooms and Cleanroom Flooring
The company plans to reduce its workforce by 53% by Q3 this year. The staff layoffs are expected to cost approximately $1.2m.
Ikena reported cash reserves of $157m as of 31 March 2024, down $18m from the end of Q4 last year.
Ikena has also discontinued developing its selective Hippo pathway inhibitor, IK-930, and will start “winddown activities”. The company added it will seek “strategic options” for the programme, including partnership agreements that could explore the “development of IK-930 in combination with other targeted agents”.

Preview
Source: Pharmaceutical Technology
Bristol Myers Squibb’s Breyanzi gains FDA approval for lymphoma

Preview
Source: Pharmaceutical Technology
IK-930 is a selective Hippo pathway inhibitor that targets TEA domain transcription factor 1 (TEAD1). The drug was being investigated in a Phase I trial (NCT05228015) in patients with solid tumours. The company planned to focus its development on treating epithelioid haemangioendothelioma (EHE) and mesothelioma.
Multiple pharmaceutical companies have announced layoffs in recent months amid a difficult economic environment. Last month, Novartis announced plans to lay off approximately 680 staff members. The plans were separate from the company’s overall restructuring programme, which could involve up to 8,000 workers.
Xilio Therapeutics also dismissed 21% of its staff in April. Additionally, the company terminated the development of XTX202, a tumour-activated beta-gamma biased interleukin (IL)-2. Similar to Ikena, Xilio did not outright dismiss the development of the therapy but instead stated that it would explore partnerships to develop the therapy as a combination treatment.
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Indications
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.