MLB Announces IND Approval by US FDA to Initiate Phase 1 Trial of TML-6, a New Era Multi-Target Drug for the Treatment of Alzheimer's Disease
07 Jun 2024
Phase 2INDPhase 1
TAINAN, June 7, 2024 /PRNewswire/ -- MLB (Merry Life Biomedical Company, Ltd., Taiwan: https://www.tmlbio.com/), a biomedical company, announced that the U.S. Food and Drug Administration (FDA) has approved IND application for
TML-6, an novel drug to treat
Alzheimer's disease (AD), enabling a Phase 1 clinical trial to be initiated this July. Advancing TML-6 into clinical trial is a critical milestone for MLB to develop a new era
multi-target drug for AD since 2018.
About TML-6
Continue Reading
Preview
Source: PRNewswire
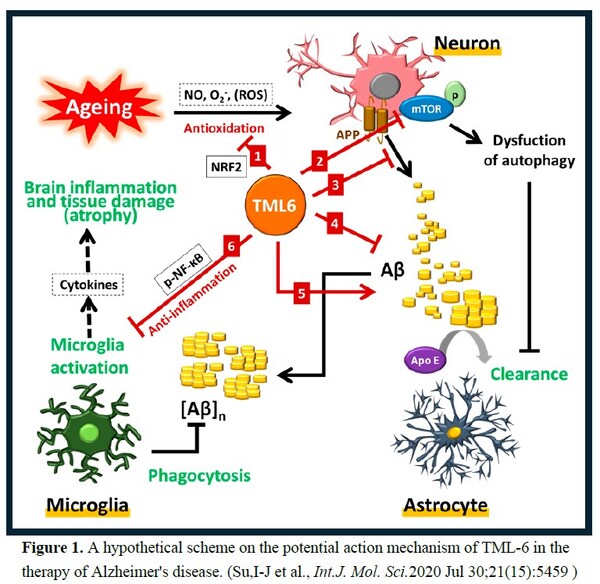
TML-6 is a novel synthetic curcumin analog. Professor Ih-Jen Su at Southern Taiwan University used a platform of 6 aging and AD biomarkers to screen the 12 compounds from Androscience (San Diego, USA) for AD candidate. Preclinical studies showed that
TML-6 (ASC-6) exhibits a
multi-target action mechanism for AD, including anti-aging, activation of autophagy (mTOR inhibitormTOR inhibitor), reducing amyloid accumulation, and anti-inflammation (Figure 1), the efficacy confirmed by 2 AD animal models.
TML-6 has a
high bioavailability through formulation and then completed preclinical toxicology and safety studies.
TML-6 should be potentially a novel drug to improve or
reverse the progression of early-stage AD.
The Design and Future Plan of TML-6 for AD Clinical Trial
s
TML-6 is developed as an oral drug and will conduct a SAD/MAD phase 1 clinical trial at Glendale Adventist Medical Center, LA, USA in 2024 Q3. Elderly cohort and CSF pharmacokinetics (PK) studies were specifically designed. For a global multi-site phase 2a clinical trial, several distinguished global AD experts provided consultation.
Blood biomarkers will be included in the phase 2a trial as the surrogate endpoint of efficacy. Furthermore, TML-6 is considering to combine with the current anti-amyloid drugs in phase 2 trial. The
drug combination could not only exhibit synergistic effects to improve AD behavior, reducing amyloid accumulation and ant-inflammation, but can also reduce antibody dosing to only 10% and avoid the adverse events (ARIAs) of anti-body drugs. MLB has successfully raised funds to conduct this global phase 2a clinical trial, scheduled to be conducted on 2025 Q3.
Contact:
Chien Hong Lin, PhD
Email: [email protected]
For more details,please visit the original website
The content of the article does not represent any opinions of Synapse and its affiliated companies. If there is any copyright infringement or error, please contact us, and we will deal with it within 24 hours.
Organizations
Indications
Targets
Hot reports
Get started for free today!
Accelerate Strategic R&D decision making with Synapse, PatSnap’s AI-powered Connected Innovation Intelligence Platform Built for Life Sciences Professionals.
Start your data trial now!
Synapse data is also accessible to external entities via APIs or data packages. Leverages most recent intelligence information, enabling fullest potential.




