/ CompletedNot Applicable 苯磺酸左氨氯地平片在中国健康受试者中进行的单中心、随机、开放、单次(空腹/餐后)口服给药、两制剂、两序列、两周期、交叉的人体生物等效性试验。
[Translation] A single-center, randomized, open-label, single-dose (fasting/postprandial) oral bioequivalence study of levamlodipine besylate tablets was conducted in healthy Chinese subjects.
主要目的:观察中国健康受试者在空腹/餐后状态下单次口服受试制剂苯磺酸左氨氯地平片(规格:2.5mg;生产厂家:贵州联盛药业有限公司)和参比制剂苯磺酸氨氯地平片(商品名:络活喜®;规格:5mg;持证商:辉瑞制药有限公司)后的药代动力学特征,评价空腹/餐后状态下两种制剂的生物等效性。
次要目的:观察受试制剂苯磺酸左氨氯地平片和参比制剂苯磺酸氨氯地平片(络活喜®)在中国健康受试者中的安全性。
[Translation] Primary objective: To observe the pharmacokinetic characteristics of the test preparation levamlodipine besylate tablets (specification: 2.5 mg; manufacturer: Guizhou Liansheng Pharmaceutical Co., Ltd.) and the reference preparation amlodipine besylate tablets (trade name: Norvasc®; specification: 5 mg; licensee: Pfizer Pharmaceuticals Co., Ltd.) after a single oral administration in the fasting/postprandial state in healthy Chinese subjects, and to evaluate the bioequivalence of the two preparations in the fasting/postprandial state.
Secondary objective: To observe the safety of the test preparation levamlodipine besylate tablets and the reference preparation amlodipine besylate tablets (Norvasc®) in healthy Chinese subjects.
以甲氨蝶呤片为基础治疗评价防薏青风湿颗粒治疗类风湿性关节炎湿热阻络证有效性和安全性临床研究。
[Translation] A clinical study was conducted to evaluate the effectiveness and safety of Fangyiqing Fengshi Granules in the treatment of rheumatoid arthritis with damp-heat obstruction of the collaterals, using methotrexate tablets as the basis for treatment.
探索防薏青风湿颗粒与甲氨蝶呤片治疗类风湿关节炎(湿热阻络证)的联合效应,并做剂量探索。观察防薏青风湿颗粒与甲氨蝶呤片联合应用的安全性。
[Translation] To explore the combined effect of Fangyiqing Fengshi Granule and methotrexate tablets in the treatment of rheumatoid arthritis (damp-heat obstruction of collaterals syndrome), and to explore the dose. To observe the safety of the combined use of Fangyiqing Fengshi Granule and methotrexate tablets.
/ Not yet recruitingNot Applicable [Translation] Study on the bioequivalence of diclofenac diethylamine emulsion in healthy volunteers
主要目的:
比较空腹给药条件下,贵州联盛药业有限公司提供的双氯芬酸二乙胺乳胶剂(规格:1%(20 g:0.2 g,以C14H10Cl2NNaO2计))与GSK Consumer Healthcare Schweiz AG持证的双氯芬酸二乙胺乳胶剂(商品名:扶他林®;规格:1%(20 g:0.2 g,以C14H10Cl2NNaO2计))在中国健康人群吸收程度和吸收速度的差异。
次要目的:
评价空腹给药条件下,贵州联盛药业有限公司提供的双氯芬酸二乙胺乳胶剂(规格:1%(20 g:0.2 g,以C14H10Cl2NNaO2计))与GSK Consumer Healthcare Schweiz AG持证的双氯芬酸二乙胺乳胶剂(商品名:扶他林®;规格:1%(20 g:0.2 g,以C14H10Cl2NNaO2))在中国健康人群体内的安全性。
[Translation] Main purpose:
To compare the differences in absorption degree and absorption rate between diclofenac diethylamine emulsion (specification: 1% (20 g: 0.2 g, calculated as C14H10Cl2NNaO2)) provided by Guizhou Liansheng Pharmaceutical Co., Ltd. and diclofenac diethylamine emulsion (trade name: Voltaren®; specification: 1% (20 g: 0.2 g, calculated as C14H10Cl2NNaO2)) certified by GSK Consumer Healthcare Schweiz AG in healthy Chinese population under fasting conditions.
Secondary objective: To evaluate the safety of diclofenac diethylamine emulsion (specification: 1% (20 g:0.2 g, calculated as C14H10Cl2NNaO2)) provided by Guizhou Liansheng Pharmaceutical Co., Ltd. and diclofenac diethylamine emulsion (trade name: Voltaren®; specification: 1% (20 g:0.2 g, calculated as C14H10Cl2NNaO2)) certified by GSK Consumer Healthcare Schweiz AG in healthy Chinese population under fasting conditions.
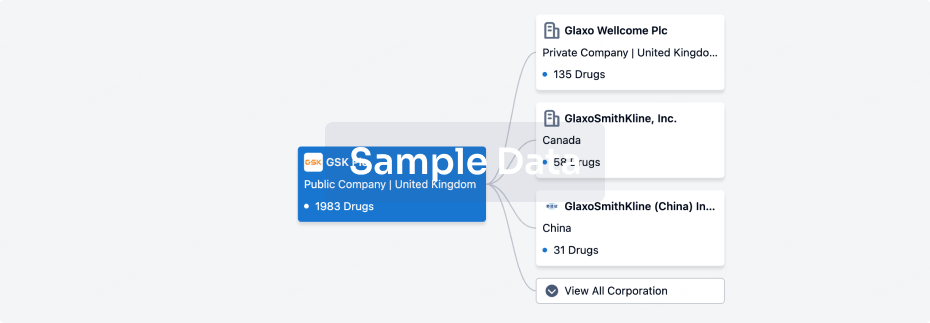
100 Clinical Results associated with Guizhou Liansheng Pharmaceutical Co., Ltd.
0 Patents (Medical) associated with Guizhou Liansheng Pharmaceutical Co., Ltd.
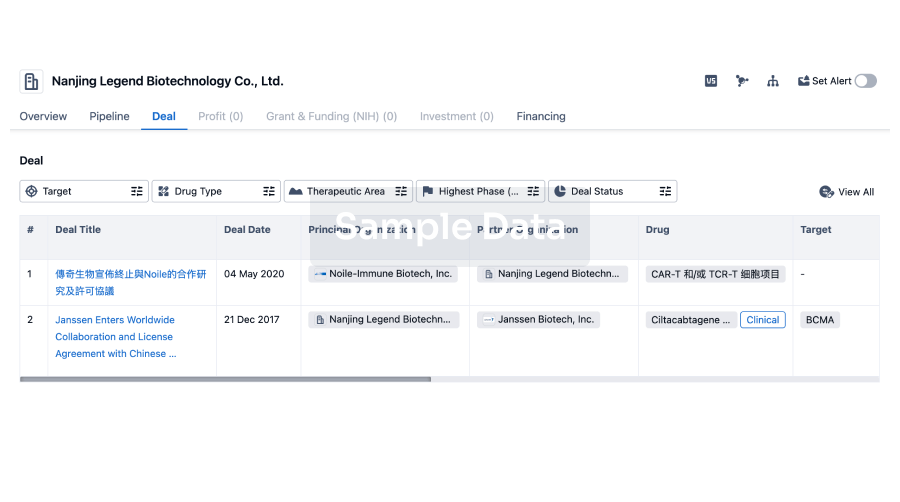
100 Deals associated with Guizhou Liansheng Pharmaceutical Co., Ltd.
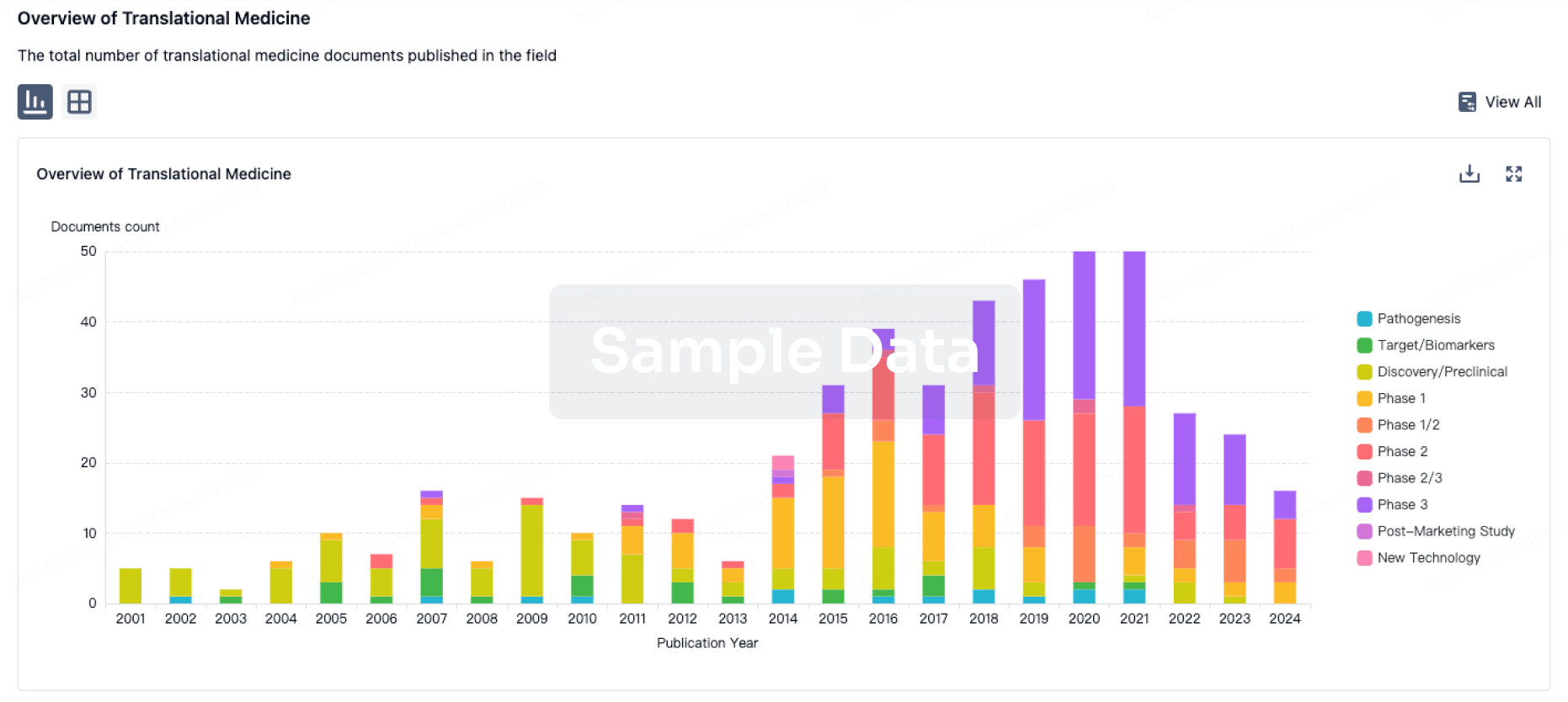
100 Translational Medicine associated with Guizhou Liansheng Pharmaceutical Co., Ltd.