/ CompletedNot Applicable [Translation] Bioequivalence study of Eperisone Hydrochloride Tablets
主要研究目的
评价中国健康成年受试者空腹条件下单次单剂量口服盐酸乙哌立松片受试制剂(商品名:宜宇,规格:50mg,申办者:青岛国海生物制药有限公司)和参比制剂(商品名:妙纳®,规格:50mg,持证商:卫材(中国)药业有限公司)后的药代动力学特点和生物等效性。
次要研究目的
研究盐酸乙哌立松片受试制剂(商品名:宜宇,规格:50mg)和参比制剂(商品名:妙纳®,规格:50mg)在中国健康成年受试者中的安全性。
[Translation] Main study objectives
To evaluate the pharmacokinetic characteristics and bioequivalence of the test formulation of Eperisone Hydrochloride Tablets (trade name: Yiyu, specification: 50mg, applicant: Qingdao Guohai Biopharmaceutical Co., Ltd.) and the reference formulation (trade name: Miaona®, specification: 50mg, licensee: Eisai (China) Pharmaceutical Co., Ltd.) after a single oral dose of Eperisone Hydrochloride Tablets in healthy Chinese adult subjects under fasting conditions.
Secondary study objectives
To study the safety of the test formulation of Eperisone Hydrochloride Tablets (trade name: Yiyu, specification: 50mg) and the reference formulation (trade name: Miaona®, specification: 50mg) in healthy Chinese adult subjects.
/ CompletedNot Applicable [Translation] Bioequivalence study of Eperisone Hydrochloride Tablets
主要研究目的
评价中国健康成年受试者空腹及餐后条件下单次单剂量口服盐酸乙哌立松片受试制剂(商品名:宜宇,规格:50mg,申办者:青岛国海生物制药有限公司)和参比制剂(商品名:妙纳®,规格:50mg,持证商:卫材(中国)药业有限公司)后的药代动力学特点和生物等效性。
次要研究目的
研究盐酸乙哌立松片受试制剂(商品名:宜宇,规格:50mg)和参比制剂(商品名:妙纳®,规格:50mg)在中国健康成年受试者中的安全性。
[Translation] Main study objectives
To evaluate the pharmacokinetic characteristics and bioequivalence of the test formulation of Eperisone Hydrochloride Tablets (trade name: Yiyu, specification: 50mg, applicant: Qingdao Guohai Biopharmaceutical Co., Ltd.) and the reference formulation (trade name: Miaona®, specification: 50mg, licensee: Eisai (China) Pharmaceutical Co., Ltd.) after a single oral dose of Eperisone Hydrochloride Tablets in healthy Chinese adult subjects under fasting and postprandial conditions.
Secondary study objectives
To study the safety of the test formulation of Eperisone Hydrochloride Tablets (trade name: Yiyu, specification: 50mg) and the reference formulation (trade name: Miaona®, specification: 50mg) in healthy Chinese adult subjects.
/ Active, not recruitingNot Applicable [Translation] Preliminary study on bioequivalence of eperisone hydrochloride tablets in human subjects
1、初步比较受试者在空腹/餐后状态下分别口服给与受试制剂和参比制剂后的药代动力学情况;2、初步考察采血点设计合理性、验证分析检测方法的重现性和适用性;3、获得盐酸乙哌立松在中国健康成人受试者中的个体内变异系数,为正式试验受试者例数提供理论依据。
[Translation] 1. To make a preliminary comparison of the pharmacokinetic profile of the test preparation and the reference preparation after oral administration in the fasting and postprandial states; 2. To preliminarily examine the rationality of the design of the blood sampling points, and verify the reproducibility and applicability of the analytical detection methods; 3. To obtain the intra-individual coefficient of variation of eperisone hydrochloride in healthy adult subjects in China, and to provide a theoretical basis for the number of subjects in the formal trial.
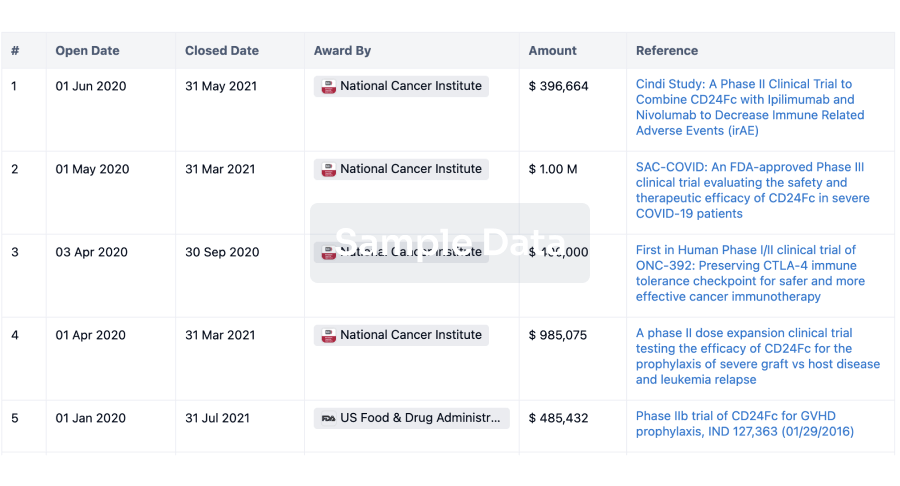
100 Clinical Results associated with Qingdao Guohai Bio-Pharmaceutical Co., Ltd.
0 Patents (Medical) associated with Qingdao Guohai Bio-Pharmaceutical Co., Ltd.
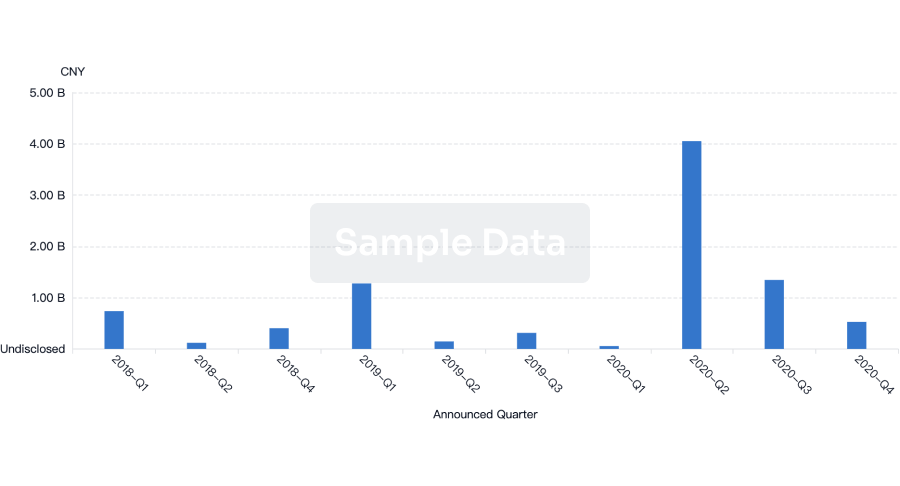
100 Deals associated with Qingdao Guohai Bio-Pharmaceutical Co., Ltd.
100 Translational Medicine associated with Qingdao Guohai Bio-Pharmaceutical Co., Ltd.