/ CompletedNot Applicable 氢溴酸替格列汀片(20mg)在中国健康受试者中空腹和餐后给药条件下随机、开放、单剂量、两序列、两周期、双交叉生物等效性试验
[Translation] A randomized, open-label, single-dose, two-sequence, two-period, double-crossover bioequivalence study of tenegliptin hydrobromide tablets (20 mg) in Chinese healthy subjects under fasting and fed conditions
主要研究目的:按有关生物等效性试验的规定,选择田辺三菱製薬株式会社持证的氢溴酸替格列汀片(商品名:TENELIA®;规格:20mg(以替格列汀计))为参比制剂,对江苏谦仁生物科技有限公司生产并提供的受试制剂氢溴酸替格列汀片(规格:20mg(按 C22H30N6OS 计))进行空腹和餐后给药人体生物等效性试验,比较受试制剂中药物的吸收速度和吸收程度与参比制剂的差异是否在可接受的范围内,评价两种制剂在空腹和餐后给药条件下的生物等效性。
次要研究目的:观察健康受试者口服受试制剂氢溴酸替格列汀片(规格:20mg(按C22H30N6OS计))和参比制剂氢溴酸替格列汀片(商品名:TENELIA®;规格:20mg(以替格列汀计))的安全性。
[Translation] The main research purpose: According to the regulations on bioequivalence testing, select the certified texagliptin hydrobromide tablets (trade name: TENELIA®; specifications: 20 mg (calculated as texagliptin) from Mitsubishi Tanbei Co., Ltd. ) is a reference preparation, and the human bioequivalence of fasting and postprandial administration was conducted on the test preparation ticagliptin hydrobromide tablets (specification: 20 mg (calculated as C22H30N6OS)) produced and provided by Jiangsu Qianren Biotechnology Co., Ltd. A safety test is conducted to compare whether the difference in absorption rate and degree of drug in the test preparation and the reference preparation is within an acceptable range, and to evaluate the bioequivalence of the two preparations under fasting and postprandial administration conditions.
Secondary research purpose: To observe the oral administration of the test preparation Telagliptin Hydrobromide Tablets (specification: 20 mg (calculated as C22H30N6OS)) and the reference preparation Telagliptin Hydrobromide Tablets (trade name: TENELIA®) to healthy subjects. ; Specification: 20mg (calculated as texagliptin)) safety.
/ CompletedNot Applicable 江苏谦仁生物科技有限公司的利奥西呱片与Bayer AG的利奥西呱片(商品名:安吉奥)在健康男性受试者中的单次给药、随机、开放、两周期交叉、空腹及餐后状态下的生物等效性研究
[Translation] A single-dose, randomized, open-label, two-period crossover, fasting and fed bioequivalence study of Riociguat tablets from Jiangsu Qianren Biotechnology Co., Ltd. and Riociguat tablets (trade name: Angio) from Bayer AG in healthy male subjects
主要研究目的:以江苏谦仁生物科技有限公司的利奥西呱片为受试制剂,以Bayer AG持证的利奥西呱片为参比制剂,通过单中心、随机、开放、单次给药、两周期交叉临床研究来评价两种制剂空腹及餐后状态下的人体生物等效性。
次要研究目的:观察受试制剂和参比制剂在中国健康受试者中的安全性。
[Translation] The main purpose of the study is to use Riociguat tablets produced by Jiangsu Qianren Biotechnology Co., Ltd. as the test preparation and Riociguat tablets certified by Bayer AG as the reference preparation. The bioequivalence of the two preparations in the fasting and fed state is evaluated through a single-center, randomized, open, single-dose, two-period crossover clinical study.
Secondary purpose of the study: To observe the safety of the test preparation and the reference preparation in healthy Chinese subjects.
/ CompletedNot Applicable 西格列汀二甲双胍缓释片在空腹条件下的人体内生物等效性预试验
[Translation] Preliminary bioequivalence study of sitagliptin and metformin extended-release tablets in humans under fasting conditions
主要研究目的
评价中国健康成年受试者空腹条件下单次单剂量口服西格列汀二甲双胍缓释片受试制剂(规格:50mg:1000mg,申办者:江苏谦仁生物科技有限公司)和参比制剂(商品名:Janumet®XR,规格:50mg:1000mg,持证商:Merck Sharp and Dohme LLC)后的药代动力学特点和生物等效性。
次要研究目的
研究西格列汀二甲双胍缓释片受试制剂(规格:50mg:1000mg)和参比制剂(商品名:Janumet®XR,规格:50mg:1000mg)在中国健康成年受试者中的安全性。
[Translation] Main study objectives
To evaluate the pharmacokinetic characteristics and bioequivalence of the test formulation of sitagliptin metformin extended-release tablets (specifications: 50mg:1000mg, applicant: Jiangsu Qianren Biotechnology Co., Ltd.) and the reference formulation (trade name: Janumet®XR, specifications: 50mg:1000mg, licensee: Merck Sharp and Dohme LLC) after a single oral dose under fasting conditions in healthy Chinese adult subjects.
Secondary study objectives
To study the safety of the test formulation of sitagliptin metformin extended-release tablets (specifications: 50mg:1000mg) and the reference formulation (trade name: Janumet®XR, specifications: 50mg:1000mg) in healthy Chinese adult subjects.
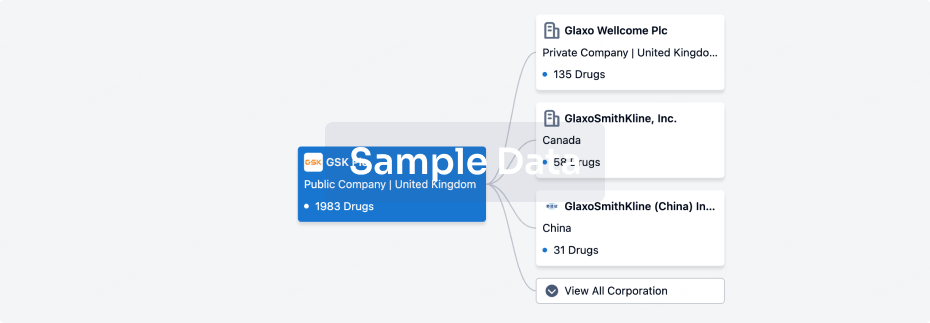
100 Clinical Results associated with Jiangsu Qianren Biotechnology Co., Ltd.
0 Patents (Medical) associated with Jiangsu Qianren Biotechnology Co., Ltd.
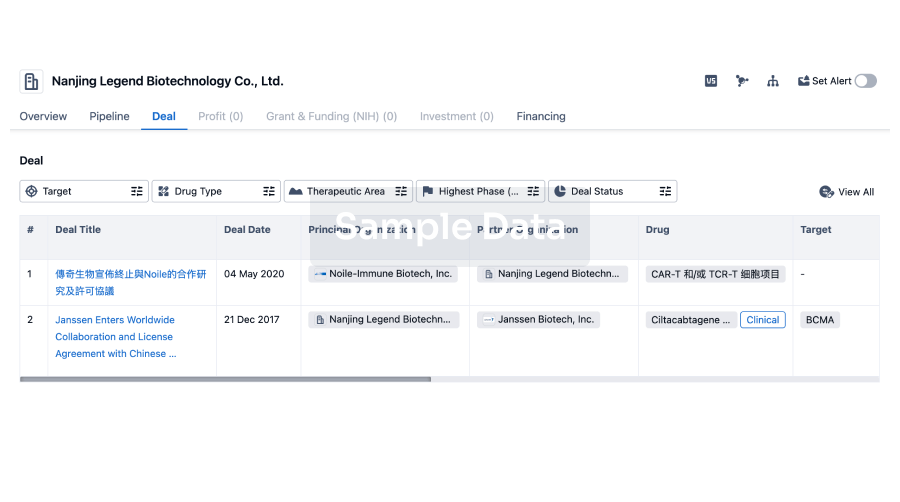
100 Deals associated with Jiangsu Qianren Biotechnology Co., Ltd.
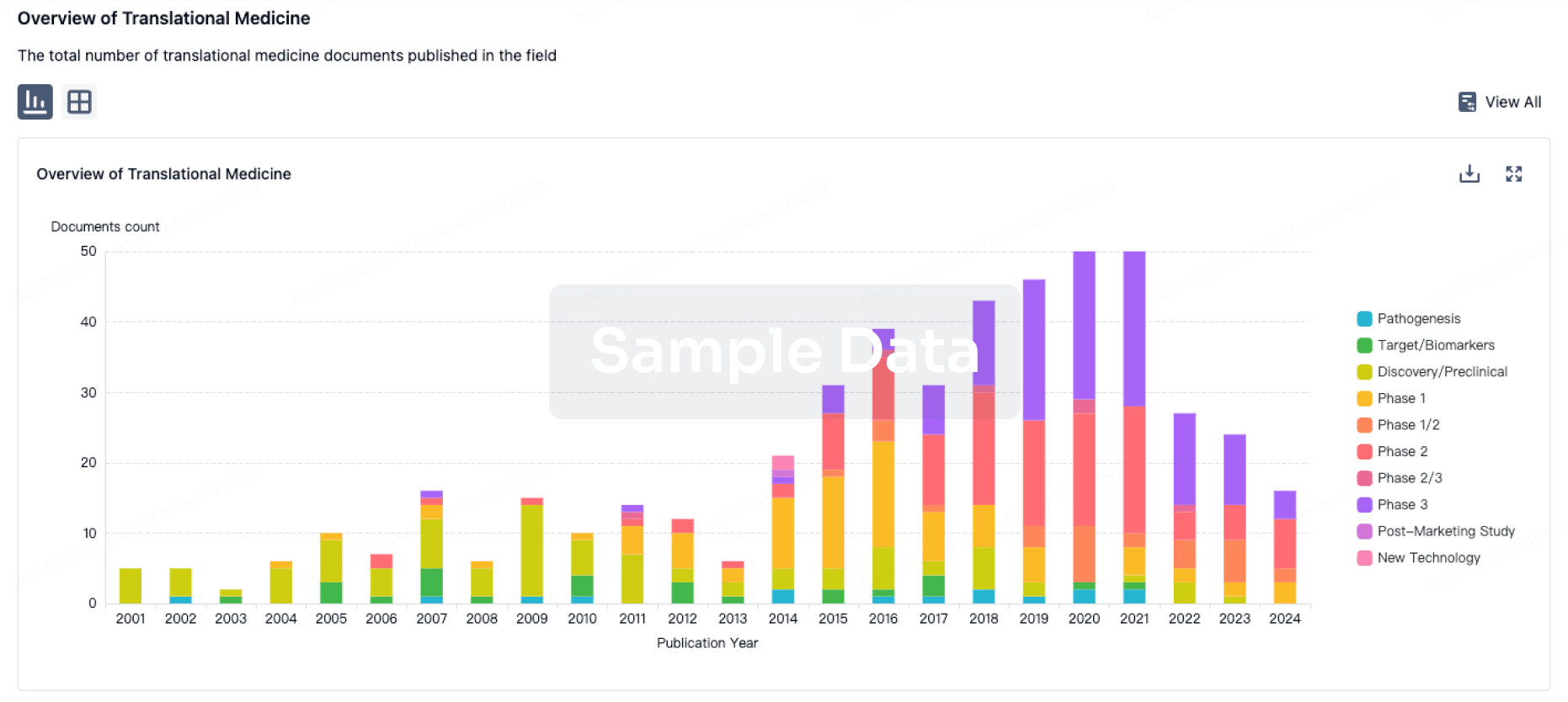
100 Translational Medicine associated with Jiangsu Qianren Biotechnology Co., Ltd.