/ CompletedNot Applicable 屈螺酮炔雌醇片 在中国健康志愿者中空腹及餐后状态下的人体生物等效性试验
[Translation] Human bioequivalence study of drospirenone and ethinyl estradiol tablets in Chinese healthy volunteers under fasting and fed conditions
主要目的:研究空腹和餐后状态下单次口服受试制剂屈螺酮炔雌醇片(规格:每片含炔雌醇0.03mg和屈螺酮3mg,乐福思健康产业股份公司生产)与参比制剂屈螺酮炔雌醇片(优思明®,规格:每片含炔雌醇0.03mg和屈螺酮3mg;Bayer Australia Ltd持证,Bayer Weimar GmbH & Co. KG.生产)在健康成年受试者体内的药代动力学特征,评价空腹和餐后状态下口服两种制剂的生物等效性。
次要目的:研究受试制剂屈螺酮炔雌醇片(规格:每片含炔雌醇0.03mg和屈螺酮3mg)和参比制剂屈螺酮炔雌醇片(优思明®)(规格:每片含炔雌醇0.03mg和屈螺酮3mg)在健康受试者中的安全性。
[Translation] Primary objective: To study the pharmacokinetic characteristics of the test preparation drospirenone ethinyl estradiol tablets (specification: each tablet contains 0.03 mg ethinyl estradiol and 3 mg drospirenone, produced by Love Health Industry Co., Ltd.) and the reference preparation drospirenone ethinyl estradiol tablets (Yasmin®, specification: each tablet contains 0.03 mg ethinyl estradiol and 3 mg drospirenone; licensed by Bayer Australia Ltd, produced by Bayer Weimar GmbH & Co. KG.) in healthy adult subjects after single oral administration in fasting and fed state, and to evaluate the bioequivalence of the two preparations after oral administration in fasting and fed state.
Secondary objective: To study the safety of the test preparation drospirenone ethinyl estradiol tablets (specification: each tablet contains 0.03 mg ethinyl estradiol and 3 mg drospirenone) and the reference preparation drospirenone ethinyl estradiol tablets (Yasmin®) (specification: each tablet contains 0.03 mg ethinyl estradiol and 3 mg drospirenone) in healthy subjects.
/ CompletedNot Applicable [Translation] Bioequivalence study of levonorgestrel tablets
主要试验目的:在健康女性受试者体内,分别在空腹和餐后条件下,以Gedeon Richter Plc.持有的左炔诺孕酮片(规格:1.5 mg,商品名:保仕婷®)为参比制剂,研究乐福思健康产业股份公司研制的左炔诺孕酮片(规格:1.5 mg;受试制剂)的药代动力学,评价受试制剂与参比制剂是否具有生物等效性。 次要试验目的:观察健康女性受试者口服受试制剂、参比制剂的安全性。
[Translation] The main purpose of the study is to study the pharmacokinetics of levonorgestrel tablets (specification: 1.5 mg; test preparation) developed by Love Health Industry Co., Ltd. in healthy female subjects under fasting and postprandial conditions, using levonorgestrel tablets (specification: 1.5 mg, trade name: Baoshiting®) held by Gedeon Richter Plc. as the reference preparation, and to evaluate whether the test preparation and the reference preparation are bioequivalent. The secondary purpose of the study is to observe the safety of oral administration of the test preparation and the reference preparation in healthy female subjects.
100 Clinical Results associated with Wuhan Jissbon Sanitary Product Co. Ltd.
0 Patents (Medical) associated with Wuhan Jissbon Sanitary Product Co. Ltd.
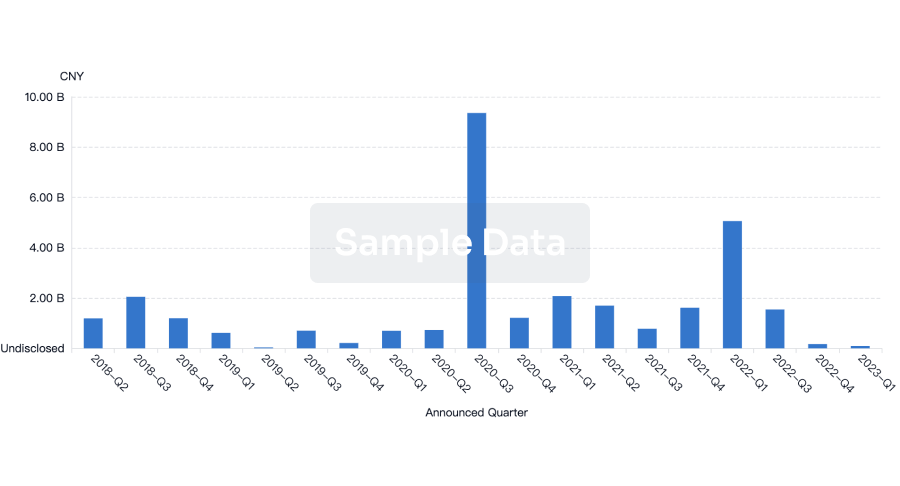
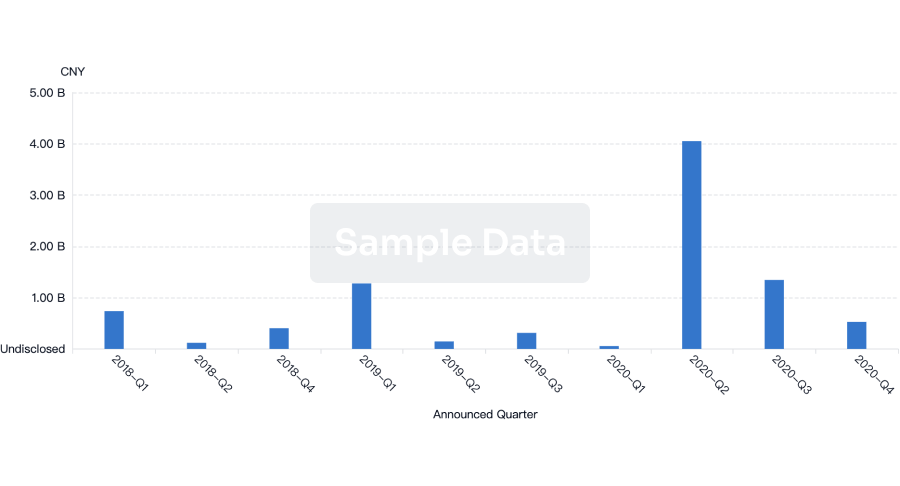
100 Deals associated with Wuhan Jissbon Sanitary Product Co. Ltd.
100 Translational Medicine associated with Wuhan Jissbon Sanitary Product Co. Ltd.